Abstract
The homeostatic blood protease, activated protein C (APC), can function as (1) an antithrombotic on the basis of inactivation of clotting factors Va and VIIIa; (2) a cytoprotective on the basis of endothelial barrier stabilization and anti-inflammatory and antiapoptotic actions; and (3) a regenerative on the basis of stimulation of neurogenesis, angiogenesis, and wound healing. Pharmacologic therapies using recombinant human and murine APCs indicate that APC provides effective acute or chronic therapies for a strikingly diverse range of preclinical injury models. APC reduces the damage caused by the following: ischemia/reperfusion in brain, heart, and kidney; pulmonary, kidney, and gastrointestinal inflammation; sepsis; Ebola virus; diabetes; and total lethal body radiation. For these beneficial effects, APC alters cell signaling networks and gene expression profiles by activating protease-activated receptors 1 and 3. APC’s activation of these G protein–coupled receptors differs completely from thrombin’s activation mechanism due to biased signaling via either G proteins or β-arrestin-2. To reduce APC-associated bleeding risk, APC variants were engineered to lack >90% anticoagulant activity but retain normal cell signaling. Such a neuroprotective variant, 3K3A-APC (Lys191-193Ala), has advanced to clinical trials for ischemic stroke. A rich data set of preclinical knowledge provides a solid foundation for potential translation of APC variants to future novel therapies.
Introduction
The body employs a variety of mechanisms to maintain tissue homeostasis and minimize damage from excessive activation of the multiple host defense system components. The protein C system provides negative-feedback regulation of host defense systems and can promote balanced regeneration of key tissue components. These mechanisms are evident from many basic laboratory studies, preclinical data, and clinical observations.1-8 The groundwork for understanding the protein C system in humans initially came from the discovery of severe protein C deficiency linked to neonatal purpura fulminans,9 which is fatal unless treated aggressively,10 and of mild heterozygous protein C deficiency linked to increased risk for venous thrombosis.11 Subsequently, targeted deletion of the murine protein C gene was found to cause perinatal lethality with pathological lesions in the brain.12,13 Thus, both human and murine data prove protein C’s essential physiological roles as an antithrombotic and anti-inflammatory protein.
The protein C system’s antithrombotic mechanisms and its utility for treating sepsis have been studied for decades. However, the remarkable ability of activated protein C (APC), a trypsinlike serine protease, to initiate beneficial cell signaling is a recent topic for investigation.1,5,14-16 APC initiates cell signaling that drives multiple, diverse, independent types of cellular activities, many of which are termed cytoprotective activities, comprising antiapoptotic and anti-inflammatory activities, favorable alterations of gene expression, and stabilization of endothelial barriers. Furthermore, APC-initiated signaling can favorably drive cell proliferation and differentiation, as well as angiogenesis, thus defining actions of APC that make it attractive for pharmacologic therapies in which cell and tissue regeneration is important, such as for neurogenesis in the setting of ischemic stroke17,18 and for wound healing.14
This review emphasizes APC’s cytoprotective properties involving biased signaling via protease-activated receptor (PAR)1, which provide benefits in multiple preclinical animal injury models. As reviewed herein, there is a plethora of data implying that wild-type (wt)-APC and/or APC variants merit development for translation to the clinic.
The protein C pathways
APC: activation, cellular signaling activity, antithrombotic activity, and inactivation
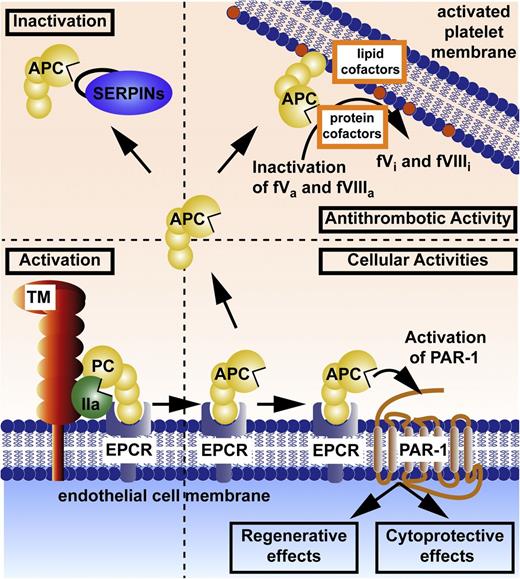
Key molecular players in the protein C system include protein C, protein S, thrombomodulin, endothelial protein C receptor (EPCR), PAR1, and PAR3.1,7 Activation of the EPCR-bound protein C zymogen is accomplished by thrombomodulin-bound thrombin (Figure 1). The active protease, APC, can dissociate from EPCR and then diffuse to other sites to interact with its substrates and receptors on cells. APC can provide 3 major types of activity: antithrombotic, cytoprotective, and regenerative (Figure 1). Anticoagulant activity is based on limited proteolytic inactivation of the activated clotting factors Va and VIIIa by APC.19 Cytoprotective actions of APC include its antiapoptotic and anti-inflammatory activities, as well as its ability to stabilize endothelial barriers to prevent vascular leakage. These cytoprotective activities generally require EPCR and involve APC’s ability to activate PAR1. APC’s signaling may also require PAR3, sphingosine-1-phosphate (S1P) receptor 1 (S1P1), Mac-1, apolipoprotein E receptor 2, epidermal growth factor receptor, Tie2, and/or other receptors (see below). The regenerative activities of APC in brain and skin require EPCR, PAR1, PAR2, and PAR3 and often S1P and S1P1.14,18,20 APC can alter expressions of hundreds of proteins, and beneficial actions of APC involve altered gene expression profiles (see Mosnier et al1 ).21,22 Additional receptors to those shown in Figure 1 may be required for APC’s regenerative properties; for example, PAR3 and S1P1 for neurogenesis,18 and PAR2, epidermal growth factor receptor, and Tie2 for wound healing.14,20 Inactivation of circulating APC by plasma serine protease inhibitors (Figure 1, upper left) is a major mechanism for clearance of APC.
Protein C activation and expression of APC’s multiple activities. Activation of the EPCR-bound protein C (PC) zymogen (bottom left) is accomplished by thrombomodulin (TM)-bound thrombin (IIa). Anticoagulant activity (upper right) is based on limited proteolysis, causing irreversible inactivation (i) of the activated clotting factors (f)Va and fVIIIa for which various lipids and protein cofactors play essential roles, as shown for this reaction on platelet membranes. Cytoprotective actions of APC (bottom right) include its antiapoptotic and anti-inflammatory activities, its ability to stabilize endothelial barriers to prevent vascular leakage, and its ability to alter gene expression profiles for many genes. APC’s various cytoprotective activities and regenerative effects generally require EPCR and PAR1. Not depicted here is the fact that APC’s cytoprotective or regenerative actions sometimes require PAR3 and/or other receptors, depending on the biological context, cell type, and organ. Inactivation of circulating APC by plasma serine protease inhibitors (SERPINs; upper left) is a major mechanism for clearance of APC. Coloring of molecules is as follows: protein C zymogen and active protease, APC (yellow); IIa (green); TM (red); EPCR (blue); and SERPINs (purple).
Protein C activation and expression of APC’s multiple activities. Activation of the EPCR-bound protein C (PC) zymogen (bottom left) is accomplished by thrombomodulin (TM)-bound thrombin (IIa). Anticoagulant activity (upper right) is based on limited proteolysis, causing irreversible inactivation (i) of the activated clotting factors (f)Va and fVIIIa for which various lipids and protein cofactors play essential roles, as shown for this reaction on platelet membranes. Cytoprotective actions of APC (bottom right) include its antiapoptotic and anti-inflammatory activities, its ability to stabilize endothelial barriers to prevent vascular leakage, and its ability to alter gene expression profiles for many genes. APC’s various cytoprotective activities and regenerative effects generally require EPCR and PAR1. Not depicted here is the fact that APC’s cytoprotective or regenerative actions sometimes require PAR3 and/or other receptors, depending on the biological context, cell type, and organ. Inactivation of circulating APC by plasma serine protease inhibitors (SERPINs; upper left) is a major mechanism for clearance of APC. Coloring of molecules is as follows: protein C zymogen and active protease, APC (yellow); IIa (green); TM (red); EPCR (blue); and SERPINs (purple).
APC: mechanisms for PAR1-mediated biased signaling
A particularly difficult conundrum arose when extensive studies showed that PAR1 mediates thrombin’s disruption of endothelial barrier, leading to vascular leakage, while paradoxically, PAR1 mediates APC’s endothelial-barrier protection, preventing vascular leakage.25,26 Similarly, PAR1 mediates some of thrombin’s proinflammatory actions, whereas PAR1 mediates APC’s antiapoptotic and anti-inflammatory activities. The solution to this conundrum is based on the ability of G protein–coupled receptors (GPCRs) to initiate entirely different signaling pathways when stimulated by different “biased” agonists.27-29
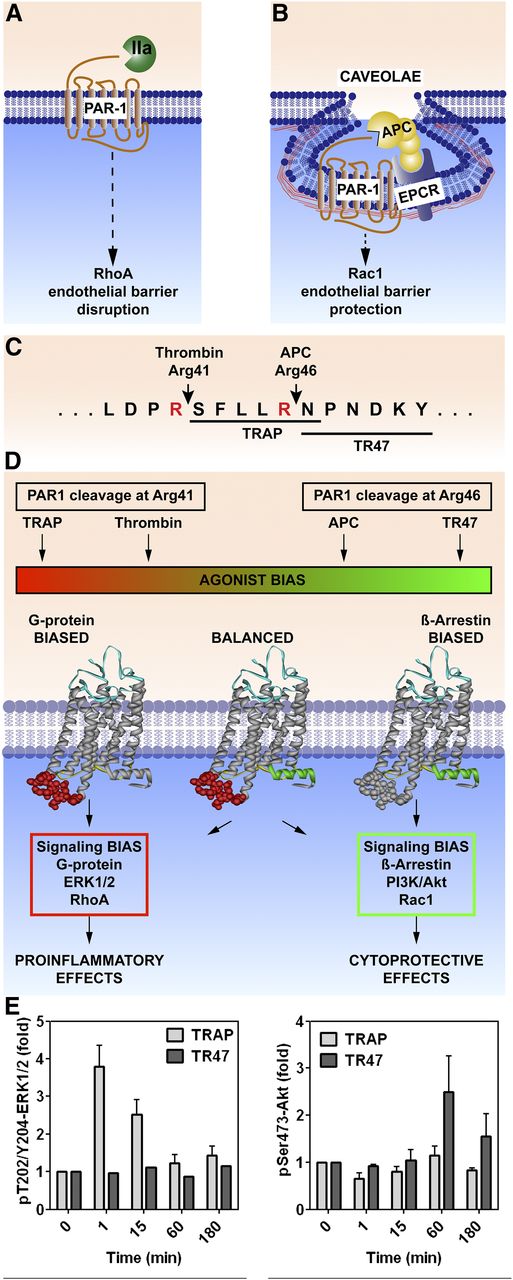
PAR1, like some other GPCRs, is capable of biased signaling, which occurs either via G proteins or via β-arrestin-2.30,31 Multiple considerations help to define and understand PAR1-biased signaling. First, different subpopulations of PAR1 are localized either in membranes that lack caveolin-1 (Figure 2A) or in caveolae microdomains that also contain EPCR (Figure 2B).32,33 Second, PAR1 signaling is initiated by different tethered N-terminal peptide sequences arising from different cleavages in the extracellular N-terminus, either the canonical Arg41 thrombin cleavage site (ie, widely recognized as the essential thrombin cleavage site) or the novel APC Arg46 cleavage site (Figure 2C).31,34,35 Third, following thrombin cleavage, PAR1 initiates signaling involving the G proteins ERK1/2 and RhoA; alternatively, following APC cleavage at Arg46, PAR1 initiates signaling involving β-arrestin-2, phosphatidylinositol 3-kinase/Akt, and Rac1 (Figure 2D). Fourth, peptides mimicking the N-terminus of cleaved PAR1 are peptide agonists with pharmacologic effects resembling those of the respective proteases that cleave PAR1 differentially. For example, a 6-mer or 10-mer TRAP that begins with Ser-42 promotes G protein–mediated signaling similar to thrombin, whereas a 20-mer peptide that begins with Asn-47 (TR47) promotes APC-like signaling (Figure 2D).31,34,36 Endothelial cell studies show that TRAP, but not TR47, promotes ERK1/2 phosphorylation, whereas TR47, but not TRAP, promotes Akt phosphorylation (Figure 2E).31 Thrombin and TRAPs cause endothelial-barrier disruption and proinflammatory effects, whereas APC and the TR47 peptide cause barrier-protective and anti-inflammatory effects (Figure 2E). Hence, the same GPCR (PAR1) is capable of biased signaling with absolutely opposing outcomes for the cell, the tissue, and the host, depending on which coagulation system (protease, thrombin, or APC) is cleaving PAR1.
PAR1-dependent biased signaling initiated by thrombin or APC. PAR1 subpopulations are localized either in membrane sections that lack caveolin-1 (A) or in caveolae that contain caveolin-1 and EPCR (B). (C) PAR1 cleavage by thrombin at Arg41 generates the N-terminal tethered-peptide agonist that begins with residue 42, whereas APC cleavage at Arg46 generates a different N-terminal tethered agonist that begins with residue 47. (D) The former cleavage results in G protein–dependent signaling, whereas the latter cleavage results in β-arrestin-2–dependent signaling. Synthetic peptides known as TRAPs that begin with amino acid 42 cause thrombinlike effects on cells, whereas a 20-mer synthetic peptide that begins with amino acid 47 (TR47) causes APC-like effects on cells .31,34,36 (E) Such effects are illustrated by the differences in phosphorylations of ERK1/2 compared to Akt, because TRAP induces phosphorylation of ERK1/2 but not Akt, whereas TR47 induces phosphorylation of Akt but not ERK1/2.31 IIa, thrombin; TRAP, thrombin receptor–activating peptide.
PAR1-dependent biased signaling initiated by thrombin or APC. PAR1 subpopulations are localized either in membrane sections that lack caveolin-1 (A) or in caveolae that contain caveolin-1 and EPCR (B). (C) PAR1 cleavage by thrombin at Arg41 generates the N-terminal tethered-peptide agonist that begins with residue 42, whereas APC cleavage at Arg46 generates a different N-terminal tethered agonist that begins with residue 47. (D) The former cleavage results in G protein–dependent signaling, whereas the latter cleavage results in β-arrestin-2–dependent signaling. Synthetic peptides known as TRAPs that begin with amino acid 42 cause thrombinlike effects on cells, whereas a 20-mer synthetic peptide that begins with amino acid 47 (TR47) causes APC-like effects on cells .31,34,36 (E) Such effects are illustrated by the differences in phosphorylations of ERK1/2 compared to Akt, because TRAP induces phosphorylation of ERK1/2 but not Akt, whereas TR47 induces phosphorylation of Akt but not ERK1/2.31 IIa, thrombin; TRAP, thrombin receptor–activating peptide.
PAR1 is subject to cleavages at residues other than Arg41 or Arg46. For example, matrix metalloproteinase-1 cleaves at Asp39 and seems to result in signaling that somewhat resembles that of thrombin.37 Elastase cleaves PAR1 at Leu45, and in vitro signaling effects differ somewhat from those of thrombin.27,38 The in vivo importance and consequences of these alternative cleavages in PAR1 by other proteases are not yet as clear as are those for the actions of thrombin and APC.
EPCR, PAR1, PAR2, and PAR3 functional interrelationships
Noncanonical PAR1 cleavage and activation by APC (Figure 2C) combined with PAR1-biased signaling via β-arrestin-2 (Figure 2D) has enabled an understanding of the selectivity and the diversity of signaling repertoires initiated by thrombin and APC. PAR3 also contributes to APC’s protective effects in the brain18,39 and kidney,40 and PAR3 also manifests functional selectivity caused by APC vs thrombin. Noncanonical PAR3 cleavage by APC at Arg41, in contrast to the canonical cleavage of PAR3 by thrombin at Lys38 (Figure 3), generates distinct N-terminal tethered-ligand sequences that modulate different signaling cascades.41,42 For example, a peptide beginning with the PAR3 residue 39 promotes PAR1-mediated ERK1/2 phosphorylation that results in endothelial-barrier disruptive effects. In contrast, a peptide beginning with PAR3 residue 42 can promote APC-like barrier-protective and vascular-protective benefits in vitro and in vivo. Although functional outcomes of noncanonical PAR1 and PAR3 peptides may sometimes overlap (eg, endothelial-barrier protection),31,41 the mechanisms of action and signaling pathways involved are clearly distinct. The APC-derived TR47 PAR1 peptide induces Akt activation (Figure 2E), but the APC-derived PAR3 tethered-ligand peptide that begins with residue 42 induces activation of Tie2, resulting in upregulation of ZO-1 and stabilization of tight junctions.31,42 Thus, the functional effects of noncanonical tethered-ligand peptides are remarkable42 and show that the diverse cellular effects of APC can arise from activation of multiple receptor complexes.
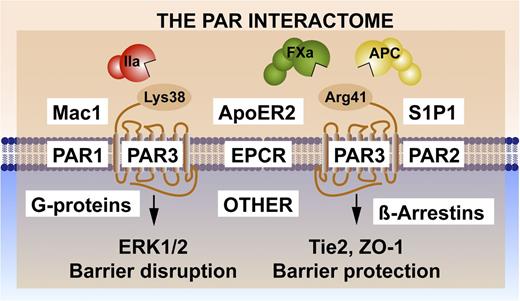
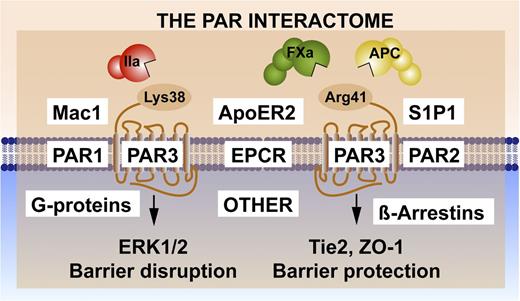
Noncanonical PAR3 activation and signaling selection by the PAR3 interactome. Activation of PAR3 can occur by thrombin (IIa)-mediated cleavage at Lys38 (canonical cleavage) or by APC and factor (F)Xa cleavage at noncanonical Arg41.41,42 The latter cleavage results in selective activation of Tie2 and ZO-1 that promotes stabilization of the tight junctions, whereas the former cleavage enhances PAR1-induced ERK1/2 activation that results in barrier-disruptive effects. Because PAR3 is considered a nonsignaling receptor, other PAR3 effectors appear to be required for signaling induction, diversification, and regulation. Collectively referred to as the PAR interactome, these components may include EPCR, which binds extracellular proteases and membrane proteins; PAR1 and/or PAR2, which can form heterodimers; other receptors such as Mac1 (CD11b/CD18), ApoER2, and Tie2, which initiate signaling; and intracellular signaling system components such as G proteins or β-arrestins.
Noncanonical PAR3 activation and signaling selection by the PAR3 interactome. Activation of PAR3 can occur by thrombin (IIa)-mediated cleavage at Lys38 (canonical cleavage) or by APC and factor (F)Xa cleavage at noncanonical Arg41.41,42 The latter cleavage results in selective activation of Tie2 and ZO-1 that promotes stabilization of the tight junctions, whereas the former cleavage enhances PAR1-induced ERK1/2 activation that results in barrier-disruptive effects. Because PAR3 is considered a nonsignaling receptor, other PAR3 effectors appear to be required for signaling induction, diversification, and regulation. Collectively referred to as the PAR interactome, these components may include EPCR, which binds extracellular proteases and membrane proteins; PAR1 and/or PAR2, which can form heterodimers; other receptors such as Mac1 (CD11b/CD18), ApoER2, and Tie2, which initiate signaling; and intracellular signaling system components such as G proteins or β-arrestins.
The PAR interactome: novel concepts for signaling selectivity and specificity
PAR3 is considered a nonsignaling receptor yet shows remarkable signaling selectivity. Thus, other PAR3-effector interactions are required for signal induction, diversification, and regulation (Figure 3). PAR-effector complexes are hypothesized to involve the formation of PAR-PAR heterodimers and homodimers,43 which may enable PAR-induced transactivation of other PARs, integrate the transactivation of other GPCRs such as S1P1,18,25,26 and incorporate cooperative cross talk with integrins such as Mac144,45 or other receptors such as ApoER246,47 or Tie2.20,42,48 Formation of these complexes may achieve a signaling bias by promoting or discouraging the association of particular G-protein ensembles,49,50 by recruiting β-arrestins,30 or by incorporating nontraditional PAR signaling pathways via transactivations such as the activation of Tie2 by noncanonical activation of PAR3.20,48 Here, we refer to the PAR interactome (Figure 3) as a collection of components whose interactions are key for the functional selectivity of APC’s beneficial cytoprotective and regenerative protease signaling. Potential GPCR interactomes are exceedingly complex; for example, proteomics identified >200 proteins pulled down with the β2-adrenergic receptor.51 The complexity of this PAR interactome increased considerably in recent years due to (1) the noncanonical activation of multiple PARs by multiple proteases; (2) the realization that both PAR1 and PAR2 are capable of biased signaling; and (3) the potential for the formation of PAR-PAR homodimers and heterodimers. Given these complexities (Figure 3), understanding the molecular mechanisms involved in the functional selectivities of the PAR-interactome signaling molecules provides unique challenges and opportunities for novel discoveries, with potential immediate relevance to targeted therapeutic strategies in a variety of diseases (Tables 1 and 252-84 ).
Directing of the PAR interactome by EPCR
EPCR plays an integral role in the PAR interactome. EPCR functions as a co-receptor that facilitates APC-mediated activation of PARs,85-87 but it can also potentially serve as an allosteric modulator88 of PAR1 signaling. This latter point became evident from the switch in thrombin’s functional selectivity from endothelial-barrier disruptive to barrier protective on “EPCR occupancy,” discovered by Rezaie.8,89 Not surprisingly, the impairment of cellular EPCR function and associated acquired functional deficiency of the protein C system due to EPCR encryption via editing of the EPCR-embedded lipid90,91 or due to EPCR targeting by Plasmodium falciparum–infected erythrocytes in severe and cerebral malaria92,93 underline the integral role of EPCR for the protein C system.7,94,95
Temporal regulation and spatial organization of the PAR interactome
Temporal regulation and spatial organization of the PAR interactome are, as of yet, largely unexplored attributes that may provide improved insight into the development of stage-specific disease progression as well as stage-specific therapeutic interventions. Temporal regulation of the PAR interactome in sepsis is illustrated by the “role reversal of PAR1,” whereby early PAR1 agonism is associated with vascular disruptive effects, but late PAR1 agonism, with a requirement for PAR2, is vascular protective.96 Spatial differentiation of the PAR interactome is evident from the requirement for colocalization of EPCR and PAR1 in caveolin-rich microdomains required for APC-mediated cytoprotective signaling.32,33 Another important distinction is the temporal fate of PAR1 after activation by APC vs thrombin. Thrombin-activated PAR1 is rapidly internalized as part of the signaling shut-off mechanisms; however, APC-activated PAR1 is retained on the cell surface.97 Explanations for prolonged membrane residency of APC-activated PAR1 may be related to (1) noncanonical activation of PAR1 resulting in different receptor conformers31 ; (2) association with other PAR interactome participants, such as EPCR that may prevent internalization and provide another potential molecular explanation for the “EPCR occupancy” phenomenon8,89 ; and/or (3) localization of PAR1 in caveolae.32,33 Although not substantiated by experimental support, the membrane residency of APC-activated PAR1 and accumulation over time are often used as explanations for why a kinetically unfavorable reaction can nevertheless have very significant functional effect.94 Regardless of the explanation(s), the regulation of the PAR interactome in time and space may provide a basis for regulation of functional selectivity of protease signaling and may provide mechanistic explanations for how PARs affect both the stage-specificity of disease progression and the success or failure of therapeutic interventions that target PARs.
APC: translation to preclinical therapies
Stimulated by discovery of hereditary mild and severe protein C deficiencies,9,11 preclinical studies initially used baboons to show that human plasma–derived wt-APC was antithrombotic and that it reduced death caused by Escherichia coli.52,53,55 Subsequently, many laboratories provided evidence of the beneficial properties of wt-APC and APC variants across a broad spectrum of preclinical animal injury models, as briefly highlighted below.
wt-APC: preclinical studies
Multiple preclinical studies employed human or recombinant murine wt-APC and injury models (Table 1). On the basis of its diversity of benefits, the breadth of APC’s effects in preclinical injury models is astounding. Pharmacologic APC promotes in vivo homeostasis and healing in the brain, heart, lungs, kidney, gastrointestinal tract, spleen, eye, bone marrow, and skin. These data for preclinical research (Table 1) establish that pharmacologic APC promotes healing and tissue homeostasis in almost every organ of the body. The breadth of APC’s pharmacologic in vivo effects mirrors its in vitro effects on endothelial cells, epithelial cells, neurons, astrocytes, keratinocytes, podocytes, dendritic cells, osteoblasts, fibroblasts, and others. The ability of APC to alter expression of hundreds of genes in different cell types is also likely key to many of APC’s benefits.
In developed countries, I/R injury is a key for the widespread morbidity and mortality of cardiovascular and cerebrovascular diseases. In multiple preclinical models of cardiac I/R injury, APC has demonstrated significant benefits.59-63 PAR1 and EPCR were required for the in vivo beneficial effects of APC in murine coronary I/R injury.63 Despite multiple studies showing reduction of coronary I/R injury, it remains to be established how long after ischemia onset APC may be beneficial or what receptors, in addition to PAR1, mediate APC’s cardioprotection. Further studies on cardioprotection by wt-APC and APC variants may well lead to translation to the clinic.
APC variants: preclinical studies
Clinical studies showed that recombinant wt-APC therapy increases risk for serious bleeding,98-100 implying that APC mutants that have reduced anticoagulant activity but retain normal cell-signaling actions should enable APC therapies with reduced risk of bleeding complications. Preclinical studies of the signaling-selective APC mutants 5A-APC, 3K3A-APC, APC-2Cys, and K193E-APC, and of an anticoagulant-selective APC mutant, E149A-APC, have proved invaluable to clarify which of APC’s 2 broad types of actions, namely anticoagulant or cytoprotective, is more important or essential for reducing injury in preclinical injury models (Table 2).81,101-105
In studies of death caused by endotoxin or bacteremia, the signaling-selective recombinant 5A-APC mutant reduced mortality, whereas the anticoagulant-selective E149A-APC mutant failed to reduce death.81,104 Mortality reduction by 5A-APC required EPCR and PAR1.81 Similarly, 5A-APC stabilized the pulmonary vasculature and reduced death caused by Pseudomonas aeruginosa, confirming a primary role for APC’s cell-signaling activities.82 Thus, the cell-signaling actions of APC involving PAR1 and EPCR are key for reducing death in murine sepsis models. Importantly, this mechanism of action contrasts with one of the central concepts that drove the development in the last century of recombinant wt-APC for sepsis therapy, which emphasized that APC would reduce organ failure by preventing or reducing consumptive coagulopathy and disseminated intravascular coagulation. Indeed, the dosing of wt-APC for multiple clinical trials for sepsis100 involved low-dose APC infusion for 96 hours, which seems rather suboptimal for therapeutic agents that act by resetting cell-signaling networks.
When the signaling-selective recombinant 5A-APC or 3K3A-APC mutants were used to treat various brain injuries, they were at least as effective as wt-APC for reducing brain injuries caused by ischemic stroke, traumatic brain injury, or amyotrophic lateral sclerosis (Tables 1 and 2). Signaling-selective variants were effective for promoting neurogenesis, emphasizing the primary role for APC’s cell-signaling activities for neuroprotection (see below).
Preclinical studies of neuroprotective activity of wt-APC and APC variants
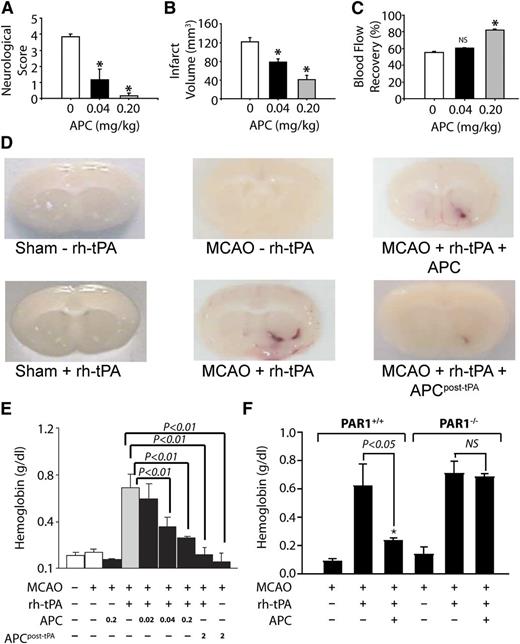
Brain preclinical injury studies have provided extensive information about and insight into APC.1-3,6,39,56-58 The neuroprotective activities of APC are seen in its ability to reduce brain damage caused by I/R injury and to reduce tissue plasminogen activator (tPA)-induced bleeding after I/R injury (Figure 4).
APC provides extensive PAR1-dependent neuroprotective effects after murine ischemia-induced stroke in the absence or presence of tPA. When APC was given 10 minutes after onset of murine middle cerebral artery occlusion (MCAO), it had beneficial effects on motor neurologic score (A), total brain infarct volume (B), and postischemic cerebral blood flow (C). (D) In MCAO studies, recombinant human (rh)-tPA was given, and tPA-induced brain hemorrhage was visualized as hemoglobin leakage at 24 hours (tPA, 0.2 mg/kg). (E) For other studies, mice received either 0.2 mg/kg APC with tPA or 2.0 mg/kg APC at 3 hours post-tPA, and bleeding in the ischemic hemisphere was quantified. Results showed that APC decreased tPA-induced bleeding. (F) Similar studies using PAR1 null mice (PAR1−/−) showed that PAR1 was required for the ability of APC to prevent tPA-induced bleeding. Panels A-C reprinted from Cheng et al57 with permission; panels D-F reprinted from Cheng et al58 with permission.
APC provides extensive PAR1-dependent neuroprotective effects after murine ischemia-induced stroke in the absence or presence of tPA. When APC was given 10 minutes after onset of murine middle cerebral artery occlusion (MCAO), it had beneficial effects on motor neurologic score (A), total brain infarct volume (B), and postischemic cerebral blood flow (C). (D) In MCAO studies, recombinant human (rh)-tPA was given, and tPA-induced brain hemorrhage was visualized as hemoglobin leakage at 24 hours (tPA, 0.2 mg/kg). (E) For other studies, mice received either 0.2 mg/kg APC with tPA or 2.0 mg/kg APC at 3 hours post-tPA, and bleeding in the ischemic hemisphere was quantified. Results showed that APC decreased tPA-induced bleeding. (F) Similar studies using PAR1 null mice (PAR1−/−) showed that PAR1 was required for the ability of APC to prevent tPA-induced bleeding. Panels A-C reprinted from Cheng et al57 with permission; panels D-F reprinted from Cheng et al58 with permission.
Despite preclinical and clinical testing of hundreds of agents for ischemic stroke therapies, only tPA has been clinically approved. Because tPA can promote hemorrhagic transformation of an ischemic stroke, it must be used within 4.5 hours of onset of stroke, limiting its efficacy. Moreover, tPA is neuronal toxic. The neuroprotective effects of APC remarkably include reduction of tPA’s neurotoxic side effects. For example, in murine ischemic stroke models, APC reduced tPA-induced bleeding in the brain (Figure 4D-E). Notably, the ability of APC to reduce tPA-induced bleeding required PAR1, which was a major indication for this receptor’s in vivo role. APC also reduced tPA-induced increases in matrix metalloproteinase-9 that contribute to stroke damage.
Studies indicate that APC directly benefits not only brain microvascular endothelial cells but also neurons. The direct in vivo neuronal protective effects of APC were shown in murine N-methyl-D-aspartate excitotoxic injury models and in vitro using cultured neurons.39,106 The ability of APC to cross the blood brain barrier due to its EPCR-dependent translocation may help explain its in vivo neuronal protective actions.107
Following seminal in vitro studies for APC’s cytoprotective actions,86,87,108 the first in vivo proof of concept for the importance of APC’s cell-signaling actions came from our studies of neuroprotection and neuronal protection that indicated PAR1, EPCR, and PAR3 were required for APC’s beneficial in vivo activities.39,57 Much subsequent work has extended the proof of concept for the central role of APC’s cell signaling for its beneficial actions on the brain.
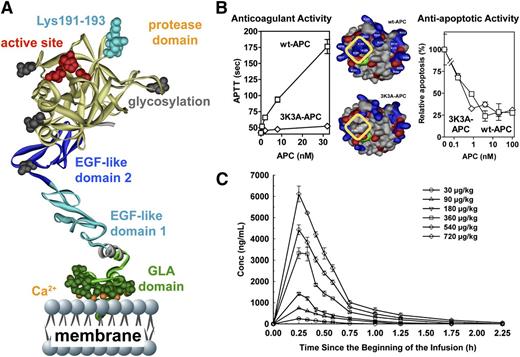
Like wt-APC, the signaling-selective 5A-APC and 3K3A-APC mutants with reduced anticoagulant activity showed neuroprotective activities that were at least as potent as those of wt-APC, and their neuroprotective abilities required PAR1 (Tables 1 and 2). For initial research using signaling-selective APC variants, we focused on murine 5A-APC that carries 5 Ala mutations because it has <10% residual anticoagulant activity. However, for translation to clinic, we focused on recombinant human 3K3A-APC, which also has <10% anticoagulant activity due to 3 Lys-to-Ala mutations in a surface-exposed loop containing Lys191-193 (Figure 5A-B).
3K3A-APC with reduced anticoagulant activity but normal cytoprotective activity is safe in humans when given as a high-dose bolus regimen. (A) The polypeptide ribbon model of APC depicts the N-terminal Gla domain at the bottom, which binds EPCR and phospholipid membranes; the EGF-1 and EGF-2 domains in the middle; and the protease domain at the top, with the “active site” triad residues (His211, Asp257, Ser360) labeled in red. Light blue coloring highlights 3 Lys residues (KKK191-193) on top of the protease domain, which form a positively charged exosite that recognizes factor Va. (B) Mutation of these Lys residues to Ala residues in the human APC variant 3K3A-APC (area outlined in yellow, middle image) deletes a factor Va binding site and consequently reduces anticoagulant activity by >90% (left graph) but does not affect 3K3A-APC’s antiapoptotic activity when tested in endothelial cell apoptosis assays (right graph). This 3K3A-APC variant is designated to be “signaling-selective” because it lacks most anticoagulant activity but retains normal cell-signaling actions. (C) In a phase 1 clinical study of 3K3A-APC, high-dose boluses were safe when administered to healthy adults and formed the basis for US Food and Drug Administration approval of a phase 2 study of 3K3A-APC in ischemic stroke patients (http://www.neuronext.org/neuronext-pleased-announce-funding-our-fourth-approved-trial-safety-evaluation-3k3a-apc-ischemic). Panel C reprinted from Lyden et al23 with permission.
3K3A-APC with reduced anticoagulant activity but normal cytoprotective activity is safe in humans when given as a high-dose bolus regimen. (A) The polypeptide ribbon model of APC depicts the N-terminal Gla domain at the bottom, which binds EPCR and phospholipid membranes; the EGF-1 and EGF-2 domains in the middle; and the protease domain at the top, with the “active site” triad residues (His211, Asp257, Ser360) labeled in red. Light blue coloring highlights 3 Lys residues (KKK191-193) on top of the protease domain, which form a positively charged exosite that recognizes factor Va. (B) Mutation of these Lys residues to Ala residues in the human APC variant 3K3A-APC (area outlined in yellow, middle image) deletes a factor Va binding site and consequently reduces anticoagulant activity by >90% (left graph) but does not affect 3K3A-APC’s antiapoptotic activity when tested in endothelial cell apoptosis assays (right graph). This 3K3A-APC variant is designated to be “signaling-selective” because it lacks most anticoagulant activity but retains normal cell-signaling actions. (C) In a phase 1 clinical study of 3K3A-APC, high-dose boluses were safe when administered to healthy adults and formed the basis for US Food and Drug Administration approval of a phase 2 study of 3K3A-APC in ischemic stroke patients (http://www.neuronext.org/neuronext-pleased-announce-funding-our-fourth-approved-trial-safety-evaluation-3k3a-apc-ischemic). Panel C reprinted from Lyden et al23 with permission.
Recombinant human 3K3A-APC was neuroprotective and extended the therapeutic window for tPA.109 It was neuroprotective when therapy was initiated beginning 4 hours after onset of ischemia and when a repeated bolus dosing regimen was employed.109,110 3K3A-APC was neuroprotective in the presence of comorbidities exemplified in hypertensive rats or in aging female mice.111 Notably, in vivo and in vitro studies showed that 3K3A-APC promoted neurogenesis in the postischemic mouse brain and in human embryo–derived neuroprogenitor cell cultures, respectively.17,18,110
APC: translation to clinical therapies
Wt-APC or 3K3A-APC has been administered to humans in the course of clinical trials targeting multiple indications that include sepsis, acute lung injury, diabetic ulcer wound healing, retinal ischemic damage, and ischemic stroke. Currently ongoing clinical studies involve ischemic stroke, wound healing, and retinal injury, whereas sepsis and acute lung injury112 are not subjects for current clinical studies.
wt-APC: approval and withdrawal for severe adult sepsis therapy
Following the successful Recombinant Human Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) trial98 in 2001 for recombinant wt-APC (drotrecogin α) for adult severe sepsis, wt-APC was approved for this indication. However, trials studying wt-APC therapy for pediatric sepsis, less-than-severe adult sepsis, and acute lung injury failed to show benefit.100,112 The PROWESS-SHOCK trial required by the European Medicines Agency was completed in 2011 and failed to show benefit for wt-APC for severe adult sepsis,113 resulting in withdrawal of the drug from the market. Nonetheless, controversy is notable, and an extensive meta-analysis and metaregression of effectiveness and safety of wt-APC for sepsis covering >40 000 patients concluded that real-life use of wt-APC was more in line with the PROWESS trial than the PROWESS-SHOCK trial (see Kalil and LaRosa99 and Christiaans et al100 ).
What are the lessons to be learned from clinical data from sepsis trials, including data for wt-APC? In the 1990s, a prevailing concept was that organ dysfunction and failure (leading to death) in sepsis were driven by intravascular coagulation, which was intimately intertwined with inflammation. Thus, APC and 2 other anticoagulants, antithrombin and tissue factor pathway inhibitor, were studied in phase III trials,98,114,115 and only the PROWESS trial was positive. Reflecting mechanistic emphasis on anticoagulation, wt-APC therapy employed a 96-hour infusion of low-dose wt-APC. But the mechanism of action for APC for sepsis in preclinical models was then not understood. Proof for the major role for APC’s cell-signaling actions (causing cytoprotective and immunoregulatory actions rather than its anticoagulant actions) came after 2006.22,44,81,102,104 Hence, a 96-hour, low-dose wt-APC infusion was not optimally suited to harness APC’s cell-signaling actions. We believe that bolus dosing is preferred to promote potent endothelial-barrier stabilization via PAR1 signaling and Rac1 activation and to alter gene expression profiles that promote antiapoptotic and anti-inflammatory activities. Future clinical studies in sepsis should be considered for signaling-selective APC variants using optimal dose(s) and timing of delivery and using careful selection of patients to identify subgroups of septic patients most likely to benefit.
3K3A-APC variant: trial for ischemic stroke therapy
Recent clinical studies are centered on the signaling-selective variant 3K3A-APC for ischemic stroke on the basis of extensive preclinical mechanism of action data that emphasize cell signaling as essential, and on the basis of greatly reduced risk of bleeding by this variant that has <10% of APC’s anticoagulant activity.109-111,116 3K3A-APC was neuroprotective and extended the therapeutic window for tPA.109 It was neuroprotective when therapy was initiated beginning 4 hours after onset of ischemia and when a repeated bolus dosing regimen was employed.109,110 3K3A-APC was neuroprotective in the presence of comorbidities exemplified in hypertensive rats or in aging female mice.111 Notably, in vivo and in vitro studies showed that 3K3A-APC promoted neurogenesis in the postischemic mouse brain and in human embryo–derived neuroprogenitor cell cultures, respectively.17,18,110
The phase I safety study in normal subjects showed that high-dose bolus regimens using 3K3A-APC are safe (Figure 5C).23 Remarkably, the very high transient levels of 3K3A-APC that were well tolerated (eg, plasma levels of 4500 ng/mL) differ markedly from sepsis therapy that provided wt-APC plasma levels of ≤50 ng/mL for 4 days. Currently, a phase II dose-escalation safety study of 3K3A-APC for ischemic stroke is underway using the National Institutes of Health–funded NeuroNEXT clinical trial network (http://www.neuronext.org/neuronext-pleased-announce-funding-our-fourth-approved-trial-safety-evaluation-3k3a-apc-ischemic).
wt-APC: studies of wound healing and ischemic retinal injury
Following an initial open-label study of wt-APC for wound healing of diabetic ulcers, Jackson and colleagues undertook a limited, double-blind study that showed significant benefits for wt-APC for diabetic ulcers, which justifies further clinical trials.14,74,117 On the basis of preclinical data showing APC benefits for I/R injury to the brain,64 Kamei and colleagues initiated ongoing open-label studies of wt-APC administered directly by injection into the eye for ischemic retinal injury, which appear promising118 and should lead to double-blind studies to clarify the real benefits of APC therapy for the ischemic damage to the retina.
Future directions
The remarkably broad range of injuries (Tables 1 and 2) for which APC or its variants provide pharmacologic benefits in preclinical studies makes it compelling to seek translation to the clinic. For various indications, knowledge is necessary about mechanisms for disease and the related mechanisms of action of APC in the context of the affected cells and organs. This knowledge should include identification of key receptors that mediate APC’s cell-signaling actions and greater insight into APC’s biased signaling mediated by PAR1 and PAR3. Development of APC variants that have preferential modes of action matched to cells, receptors, and indications may lead to improved therapies. One must avoid the pitfalls discovered for the trials of wt-APC for sepsis, whereby inadequate knowledge, albeit understandable, about mechanisms of action likely led to a suboptimal choice for effective therapy. Key considerations for both preclinical and clinical efficacy include identifying the appropriate timing and duration of therapy for APC and its variants. At this point, the challenges for translation of APC and its variants to the clinic merit full engagement.
Acknowledgments
The authors gratefully acknowledge many helpful discussions and productive interactions with our many colleagues, including especially Drs Jose A. Fernandez and Patrick D. Lyden. We apologize to our colleagues whose relevant and excellent work could not be cited due to space limitations.
This manuscript was supported, in part, by National Institutes of Health National Heart, Lung, and Blood Institute grants HL031950 and HL052246 (J.H.G.), HL063290 (B.V.Z), and HL104165 (L.O.M.).
Authorship
Contribution: J.H.G., B.V.Z., and L.O.M. wrote the manuscript.
Conflict-of-interest disclosure: J.H.G., B.V.Z., and L.O.M. are inventors for subject matters related to cytoprotective, neuroprotective APC variants. J.H.G. is a consultant for ZZ Biotech; B.V.Z. is the scientific founder of ZZ Biotech, a biotechnology company with a focus on developing APC and its functional mutants for stroke and other neurologic disorders.
Correspondence: John H. Griffin, Department of Molecular and Experimental Medicine (MEM-180), The Scripps Research Institute, 10550 North Torrey Pines Rd, La Jolla, CA 92037; e-mail: jgriffin@scripps.edu.