Key Points
Vosaroxin alone or together with LDAC does not benefit older acute myeloid leukemia patients not considered fit for intensive therapy.
In exploratory analyses, no demographic subgroup showed a survival benefit.
Abstract
The development of new treatments for older patients with acute myeloid leukemia is an active area, but has met with limited success. Vosaroxin, a quinolone-derived intercalating agent has several properties that could prove beneficial. Initial clinical studies showed it to be well-tolerated in older patients with relapsed/refractory disease. In vitro data suggested synergy with cytarabine (Ara-C). To evaluate vosaroxin, we performed 2 randomized comparisons within the “Pick a Winner” program. A total of 104 patients were randomized to vosaroxin vs low-dose Ara-C (LDAC) and 104 to vosaroxin + LDAC vs LDAC. When comparing vosaroxin with LDAC, neither response rate (complete recovery [CR]/complete recovery with incomplete count recovery [CRi], 26% vs 30%; odds ratio [OR], 1.16 (0.49-2.72); P = .7) nor 12-month survival (12% vs 31%; hazard ratio [HR], 1.94 [1.26-3.00]; P = .003) showed benefit for vosaroxin. Likewise, in the vosaroxin + LDAC vs LDAC comparison, neither response rate (CR/CRi, 38% vs 34%; OR, 0.83 [0.37-1.84]; P = .6) nor survival (33% vs 37%; HR, 1.30 [0.81-2.07]; P = .3) was improved. A major reason for this lack of benefit was excess early mortality in the vosaroxin + LDAC arm, most obviously in the second month following randomization. At its first interim analysis, the Data Monitoring and Ethics Committee recommended closure of the vosaroxin-containing trial arms because a clinically relevant benefit was unlikely.
Introduction
Almost half of patients with acute myeloid leukemia (AML) are more than 70 years of age. Population and clinical trial studies indicate that the outcome is poor, even if initial remission is achieved. Because only a minority in this age group enter clinical trials, it is difficult to assess the potential impact of any trial intervention being tested on the wider patient population. The first dilemma is what treatment approach to take. The issue here is the concern that conventional induction chemotherapy may be unsuitable for the older, less fit population, and though there is a potential for achieving remission, this will not be durable for most patients. In the past, such patients have frequently received best supportive care that did not involve any attempt to induce disease remission. An alternative is a less intensive chemotherapeutic approach in which tolerability is reasonable and hospitalization can be minimized. Currently available options include low-dose cytarabine (Ara-C) (LDAC)1-3 or a demethylation agent (such as azacitidine or decitabine), all of which have been shown to be superior to best supportive care, and between which head-to-head randomized data do not clearly demonstrate an optimal choice. However, none of these options is satisfactory at achieving or sustaining remission for most patients, and new treatments are needed.
Vosaroxin is a first-in-class anticancer quinolone derivative that targets actively replicating cells by intercalating DNA and inhibiting topoisomerase II, therefore inducing site-selective DNA double-strand breaks that result in G2 arrest and cell death through apoptosis.4 Topoisomerase II poisons, such as the anthracyclines, are already integral to the treatment of AML. Although vosaroxin is mechanistically similar to anthracyclines such as daunorubicin, its quinolone derivative scaffold provides key points of differentiation with respect to its biological action and pharmacological profile. In contrast to traditional anthracyclines, vosaroxin has a number of characteristics that might be advantageous for older patients: (1) it does not generate the significant levels of free reactive oxygen species that have been associated with anthracycline-induced cardiotoxicity5 ; (2) following IV administration, it has a favorable pharmacokinetic profile and, being minimally metabolized, clearance is predominantly through biliary excretion and intestinal secretion with a terminal half-life of 22 hours6 ; (3) it does not induce or inhibit a variety of CYP450 enzymes in vitro, suggesting low potential for clinically relevant drug interactions7 ; (4) it is not a P-glycoprotein substrate; and (5) its activity is independent of p53,8 so it thus evades 2 of the common drug resistance pathways relevant in older patients with AML.
The initial phase 1 study in patients with advanced hematological malignancies evaluated once- or twice-weekly IV administration in patients treated up to a dose of 270 mg/m2 per cycle; the dose-limiting toxicity was oral mucositis.9 Subsequent phase 2 studies have evaluated vosaroxin as a monotherapy, but our preclinical studies, which showed synergy with Ara-C, provided a rationale for combining with LDAC.7 The monotherapy doses of 72 or 90 mg/m2 were evaluated in a number of administration schedules. Of these, 72 mg/m2 on days 1 and 4 every 4 to 6 weeks achieved an overall response rate of 38%, with an acceptable 30- and 60-day mortality of 7% and 14%, respectively.10-12 This dose therefore emerged as the most suitable for further assessment.
These potential improvements in efficacy and tolerability suggested that it may be especially relevant in the clinical management of older patients with AML (generally aged older than 65 years) who frequently have resistant disease and tolerate traditional therapies poorly. We therefore prospectively investigated vosaroxin as first-line therapy for older patients with AML who were not considered fit for intensive therapy as part of the UK National Cancer Research Institute LI-1 trial, both as monotherapy and in combination with LDAC randomized with the trial standard arm of LDAC.
Methods
The aim of these studies was to compare in a 1:1 randomization of LDAC vs vosaroxin as monotherapy and LDAC vs LDAC combined with vosaroxin in older patients who were not considered suitable for intensive therapy. All centers had an intensive protocol available. These options were part of our “Pick a Winner” trial strategy13 in the LI-1 trial (ISRCTN40571019). In this trial design, patients are randomized between a control arm (LDAC) and a number of experimental options. The comparison is between each experimental option and LDAC, and not between the experimental options. Importantly, patients in the LDAC only act as controls to patients who have been contemporaneously randomized. The LDAC vs vosaroxin monotherapy randomization commenced on February 21, 2012. The monotherapy randomization opened with a “run-in” period in a limited number of centers, with enhanced pharmacovigilance involving supervision on a weekly basis. Similarly, after preliminary safety assessment of 28 randomized patients in limited centers, the second randomization of LDAC vs LDAC + vosaroxin commenced on June 28, 2012. Centers were required to have experience with vosaroxin monotherapy either in LI-1 or the Sunesis Pharmaceuticals–sponsored VALOR trial to open the combination arm. In total, 80 patients entered a 3-way randomization between LDAC or vosaroxin monotherapy or the combination, with 25 patients acting as a common control. In all, 53 patients were randomized only between vosaroxin and LDAC before the randomization closed on April 22, 2013, and 53 between LDAC + vosaroxin and LDAC from the closure of the monotherapy randomization until August 21, 2013. LDAC treatment comprised Ara-C 20 mg twice a day for 10 days by subcutaneous injection for 4 courses 4 to 6 weeks apart. Vosaroxin was given as a short IV infusion of 72 mg/m2 on days 1 and 4 of each course of LDAC with the intention to deliver 4 courses. Patients who were considered to be benefiting by demonstrating stable disease or continuing response were permitted to have more courses. Patients were required to give written consent. The trial was sponsored by Cardiff University and approved by the Wales Research Ethics Committee in compliance with the Declaration of Helsinki.
The eligibility criteria for both options were the same and included de novo and secondary AML and high-risk myelodysplastic syndrome, defined as >10% marrow blasts.
The reasons patients were not considered suitable for intensive treatment are shown in Table 1. The comorbidity assessment using the Sorror index components14 were collected at entry. Patients were categorized using the validated multiparameter Wheatley risk score,15 which is based on age, performance status, cytogenetics, and de novo or secondary disease. This score has been prospectively validated in older patients treated intensively and nonintensively with LDAC. Diagnosis and response definitions described in the following sections were designated by the local investigator. Cytogenetic (a minimum of 20 metaphases) and immunophenotypic characterization were carried out in regional reference laboratories who participate in national quality assurance schemes.
Toxicity
Adverse events and toxicity were recorded as defined by the National Cancer Institute Common Toxicity Criteria, version 3.
Definitions of end points
The protocol defined complete remission (CR) as a normocellular bone marrow aspirate containing <5% leukemic blasts and showing evidence of normal maturation of other marrow elements. Persistence of myelodysplastic features did not preclude the diagnosis of CR. Although not in the original protocol, in this report, to achieve CR, patients required neutrophil recovery to ≥1.0 × 109/L and platelets to ≥100 × 109/L, without evidence of extramedullary disease. Patients who achieved CR according to the protocol, but without evidence of count recovery, are denoted here as CRi.
Following international guidelines,16 overall survival was defined as the time from randomization to death. For remitters, relapse-free survival was the time from remission (CR or CRi) until relapse or death. Survival from CR was defined as the time from CR/CRi (first report) until death. Survival percentages are quoted at 1 year.
Statistical methods
All analyses were by intention-to-treat. Categorical end points (eg, CR rates) were compared using Mantel-Haenszel tests, giving Peto odds ratios (ORs) and confidence intervals. Continuous/scale variables were analyzed by nonparametric (Wilcoxon rank-sum) tests. Time-to-event outcomes were analyzed using the log-rank test, with Kaplan-Meier survival curves. ORs and hazard ratios (HRs) lower than 1 indicated benefit for the investigational therapy (vosaroxin or vosaroxin + LDAC). Analyses were performed for each investigational arm separately vs the control of LDAC.
In addition to overall analyses, exploratory analyses were performed stratified by the randomization stratification parameters and other important variables, with suitable tests for interaction. Because of the well-known dangers of subgroup analysis, these were interpreted cautiously.
The power calculation for the trial specified that final analysis was to be performed after 340 events (deaths) had been reported. Under the rules of the Pick a Winner design, the Data Monitoring and Ethics Committee (DMEC) initially examined outcomes after response data were available for the first 100 patients in each randomization. At this point, to show sufficient promise to be carried forward, there had to be a 2.5% improvement in remission rates (CR + CRi) for the experimental arm over the control arm. At this time, the DMEC also assessed survival and toxicity as additional criteria to be satisfied, although there was no formal stopping rule for either of these end points. If the DMEC believed there was sufficient promise in the arm, the trial would continue to accrue until 100 patients were in each arm, at which point a 7.5% improvement in remission rate was required for the trial to continue to 400 patients and 340 events.
In July 2013, the DMEC carried out an outcomes assessment on the LDAC vs vosaroxin arms of the LI-1 trial (n = 104), at which point additional randomizations were suspended pending the review. The DMEC concluded that the initial hurdle of improving remission rates by 2.5% was not passed and thus recommended permanent closure of the randomization. In November 2013, the DMEC reviewed the LDAC vs LDAC + vosaroxin (n = 104) arms of the trial and also recommended closure of this randomization because, despite any improvement in remission rates, the survival data seen (based upon 56 deaths) made the desired size of benefit unlikely to be achieved. Data presented here are based upon updated follow-up to May 1, 2014.
Results
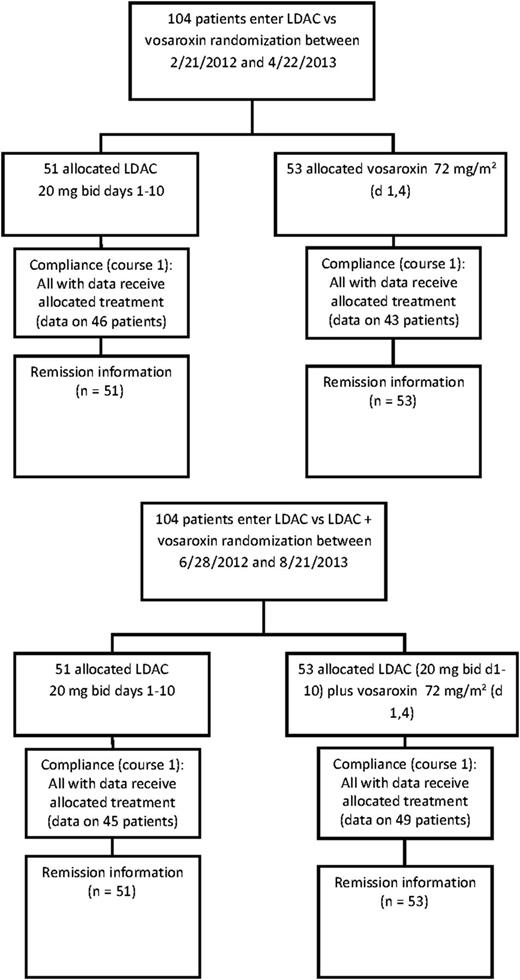
Between February 2012 and November 2013, 104 patients entered the LDAC vs vosaroxin randomization, and between June 2012 and August 2013, 104 entered the LDAC vs LDAC + vosaroxin randomization. The patient characteristics are shown in Table 1. There were no differences between the randomized arms. The median age was 75 years for both randomizations (range 60-91 years): median overall survival was 5.3 months in the vosaroxin randomization and 5.5 months in the vosaroxin combination randomization. The disposition of the patients is shown in Figure 1.
Consolidated Standards of Reporting Trials diagram. bid, twice daily; d, day.
Follow-up is complete to May 1, 2014, for patients entering both randomizations. Surviving patients are censored at the date last known to be alive. Median follow-up for the vosaroxin monotherapy randomization is 19.6 months (range 1.6-26.3 months); for the vosaroxin + LDAC randomization, it is 12.8 months (range 7.4-18.7 months).
The reasons given for not receiving intensive therapy in the vosaroxin randomization were age in 76% of cases, fitness in 40% of cases (both together in 21% of cases), and other reasons in 7% of cases, of whom half was other comorbidities and half was patient choice. In the vosaroxin combination randomization, age was a reason for not choosing intensive therapy in 89% of cases, fitness in 49% of cases (both together in 40% of cases), and other reasons in 6% of cases. Of the 104 patients randomized between LDAC and vosaroxin, the median number of treatment courses given was 3 for LDAC (mean 3.1, 0 = 4%; 1 = 33%, 2 = 12%, 3 = 12%, 4 = 14%, 5 = 10%, 6 = 8%, 7 = 0%, 8 = 8%) and 1 for vosaroxin (mean 1.5; 0 = 10%, 1 = 61%, 2 = 12%, 3 = 6%, 4 = 10%, 5 = 2%, with no patient getting more than 5 courses). Of the 104 patients randomized between LDAC and LDAC + vosaroxin, the median number of treatment courses given was 3 for LDAC (mean 3.2, 0 = 2%; 1 = 35%, 2 = 10%, 3 = 8%, 4 = 18%, 5 = 8%, 6 = 10%, 7 = 0%, 8 = 8%) and 2 for LDAC + vosaroxin (mean 2.0; 0 = 4%, 1 = 43%, 2 = 25%, 3 = 9%, 4 = 15%, 5 = 2%, 6 = 2%, with no patient getting more than 6 courses). The distribution of patients by the multiparameter risk score (Wheatley score) was 3% good risk, 36% standard risk, and 62% poor risk for vosaroxin monotherapy, and 1% good risk, 39% standard risk, and 60% poor risk for vosaroxin combined with LDAC. This validated score would predict a 12-month survival of 36%, 42%, and 14% for LDAC in the 3 risk groups. Of the comorbidities listed on entry, the most frequent were those described as cardiac in 20% of patients in the monotherapy randomization and 23% of patients in the combination randomization (Table 1).
Outcomes in the LDAC vs vosaroxin randomization
Response.
The overall response rate for all 104 patients in the monotherapy randomization was 28% (CR, 15%; CRi, 13%) and survival at 12 and 24 months was 22% and 6%, respectively. Of the 51 patients randomized to LDAC, 16% achieved CR and 14% achieved CRi, which was not significantly different from the 53 patients allocated to vosaroxin (CR, 15%; CRi, 11%; OR for CR 1.05 [0.36-3.02]; P = .9; OR for CR/CRi 1.16 [0.49-2.72]; P = .7; Table 2). The median time to recorded response was 112 days for LDAC and 45 days for vosaroxin; the median number of courses given before CR were 2.5 for LDAC and 1 for vosaroxin. There was more early mortality in the vosaroxin arm, with evidence of greater induction death (26% vs 14%; OR 2.18 [0.84-5.66]; P = .11) and significantly greater 60-day mortality (38% vs 20%; HR 2.16 [1.05-4.43]; P = .04).
Toxicity.
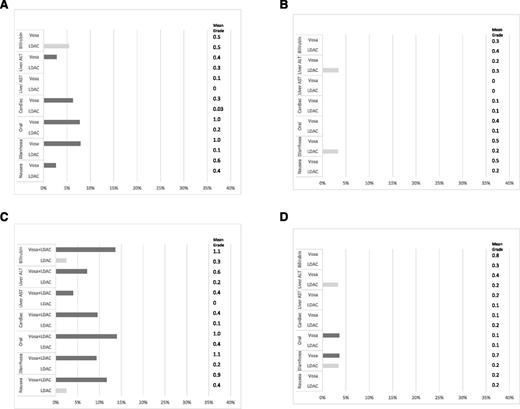
The grade 3 and 4 toxicities after course 1 for each experimental arm are shown in Figure 2A,C. Both oral toxicity and diarrhea were significantly worse for patients given vosaroxin in either alone or in combination. The level of toxicity was not increased in the combination compared with monotherapy. This gastrointestinal toxicity was not apparent in the recipients of course 2. Although the median time to recovery of neutrophils from start of course was greater in the vosaroxin arm than the LDAC arm (44 days vs 31 days), this did not reach significance (P = .16). Recipients of vosaroxin, perhaps as a consequence of gastrointestinal damage, required more days on IV antibiotics, days in hospital, and supportive transfusions. The grade 3 and 4 toxicities for course 2 are shown in Figure 2B,D. As in course 1, the vosaroxin monotherapy and vosaroxin + LDAC was associated with greater toxicity and greater resource usage (Table 3). Increased gastrointestinal toxicity was seen again in the combination arm (oral and diarrhea), although there was also a suggestion of increased liver toxicity in the combination arm. There was no evidence of greater duration of neutropenia in the vosaroxin combination arm (36 days vs 36 days, P = .7).
Grade 3/4 toxicities by course, with mean grade and test for differences using the Wilcoxon rank-sum test. (A) LDAC vs vosaroxin course 1; (B) LDAC vs vosaroxin course 2; (C) LDAC vs LDAC + vosaroxin course 1; and (D) LDAC vs LDAC + vosaroxin course 2. ALT, alanine transaminase; AST, aspartate transaminase; vosa, vosaroxin.
Grade 3/4 toxicities by course, with mean grade and test for differences using the Wilcoxon rank-sum test. (A) LDAC vs vosaroxin course 1; (B) LDAC vs vosaroxin course 2; (C) LDAC vs LDAC + vosaroxin course 1; and (D) LDAC vs LDAC + vosaroxin course 2. ALT, alanine transaminase; AST, aspartate transaminase; vosa, vosaroxin.
Outcome of nonresponders.
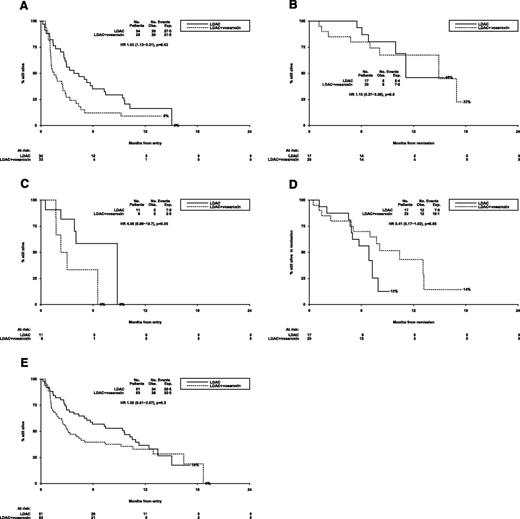
The median overall survival of patients who did not achieve CR/CRi was 2.5 months and was significantly worse in the 39 vosaroxin patients (median survival 1.9 months) compared with the 36 LDAC (median overall survival 4.9 months) (HR 1.68 [1.04-2.71; P = .03; Figure 3A).
Outcomes for patients (LDAC vs vosaroxin randomization). (A) AML LI-1: overall survival (no CR). (B) AML LI-1: overall survival from CR. (C) AML LI-1: survival from relapse. (D) AML LI-1: relapse-free survival. (E) AML LI-1: overall survival. AML LI-1, UK National Cancer Research Institute LI-1 trial; exp., expected; obs., observed.
Outcomes for patients (LDAC vs vosaroxin randomization). (A) AML LI-1: overall survival (no CR). (B) AML LI-1: overall survival from CR. (C) AML LI-1: survival from relapse. (D) AML LI-1: relapse-free survival. (E) AML LI-1: overall survival. AML LI-1, UK National Cancer Research Institute LI-1 trial; exp., expected; obs., observed.
Survival of responders.
In the 29 patients who achieved a CR/CRi, the median survival from time of response was 10.6 months, with significantly better survival for patients given LDAC compared with vosaroxin (HR 3.37 [1.17-9.67]; P = .02; Figure 3B). Achievement of a CR as opposed to a CRi was associated with longer survival from CR (median 17.5 months vs 6.9 months, P = .04; see supplemental Figure 1A on the Blood Web site).
Survival from relapse.
Following relapse, there was no significant difference between the 2 arms of the randomization (24% vs not reached for LDAC; HR 1.99 [0.59-6.63]; P = .3; Figure 3C).
Overall survival.
There was a tendency for relapse-free survival to be better in the LDAC arm (30% vs 8%; HR 2.06 [0.86-4.92]; P = .10; Figure 3D); taken together with the significant excess of early mortality, this led to significantly worse overall 12-month survival in the vosaroxin arm (31% vs 12%; OR 1.94 [1.26-3.00]; P = .003; Figure 3E). Median survival in the 2 arms was 3.2 months vs 9.0 months.
Outcomes in the LDAC vs LDAC + vosaroxin randomization
Response.
The overall response rate for all 104 patients was 36% (CR 22%; CRi 14%) and survival at 12 and 24 months was 35% and 0%, respectively. Of the 51 patients randomized to LDAC, 20% achieved CR and 14% achieved CRi, which was not significantly different from the 53 patients allocated to LDAC + vosaroxin (CR 25%; CRi 13%; OR for CR 0.75 [0.30-1.89]; P = .5; OR for CR/CRi 0.83 [0.37-1.84]; P = .6; Table 2). The median time to response was 95 days for LDAC and 55 days for vosaroxin + LDAC; the median number of courses given before CR were 2 for LDAC and 1 for vosaroxin + LDAC. There was no significant difference in 30-day mortality, which was similar between the 2 arms (11% vs 10%); however, as for the vosaroxin monotherapy there was a significantly greater 60-day mortality in the vosaroxin + LDAC arm (18% vs 36; OR 2.12 [1.01-4.45]; P = .05).
Outcome of nonresponders.
The median overall survival of patients who did not achieve CR/CRi was 2.5 months and was significantly worse for patients given LDAC + vosaroxin (median survival 1.5 months vs 4.1 months; HR 1.92 [1.1-2-3.31]; P = .02; Figure 4A).
Outcomes for patients (LDAC vs LDAC + vosaroxin randomization). (A) AML LI-1: overall survival (no CR). (B) AML LI-1: overall survival from CR. (C) AML LI-1: survival from relapse. (D) AML LI-1: relapse-free survival. (E) AML LI-1: overall survival.
Outcomes for patients (LDAC vs LDAC + vosaroxin randomization). (A) AML LI-1: overall survival (no CR). (B) AML LI-1: overall survival from CR. (C) AML LI-1: survival from relapse. (D) AML LI-1: relapse-free survival. (E) AML LI-1: overall survival.
Survival of responders.
In the 37 patients who achieved a CR/CRi, the median survival from time of response was 14.9 months, with no significant difference between arms (HR 1.15 [0.37-3.63]; P = .8; Figure 4B). The comparison of CR vs CRi was consistent with the monotherapy randomization (median not reached vs 10.1 months), although not statistically significant in this case (P = .07; supplemental Figure 1B). Following remission, treatment was given to 11 LDAC patients (3 patients received 1 course, 5 patients 2 courses, 1 patient 3 courses, and 2 patients 4 courses) and 7 patients were vosaroxin-treated (2 patients received 1 course, 4 patients received 2 courses, 1 patient received 3 courses)—in all cases, patients received their originally allocated treatment except for 1 LDAC + vosaroxin patient who received LDAC alone following CR and 1 patient who received vosaroxin monotherapy for 1 course. One patient given LDAC + vosaroxin died 25 days following the first course after remission—there were no other deaths within 30 days of the first course after remission.
Survival from relapse.
Following relapse, there was some evidence of better survival among patients treated with LDAC compared with LDAC + vosaroxin, although survival was poor in both cases, with no patients surviving more than 9 months from relapse (median survival from relapse 8.8 months vs 2.7 months; P = .05; Figure 4C).
Overall survival.
Although there was a trend for improved relapse-free survival with LDAC + vosaroxin (HR 0.41 [0.17-1.02]; P = .06; Figure 4D), the poorer outcome of patients who did not achieve remission or who relapsed meant that survival in the LDAC + vosaroxin arm was not better than for LDAC (12-month survival 33% vs 37%; median overall survival 3.1 months vs 9.6 months; HR 1.30 [0.81-2.07]; P = .3; Figure 4E).
Exploratory subgroup analysis.
Exploratory analyses were carried out on survival to find out if there was an identifiable subgroup with a differential effect of treatment. Baseline covariates including age, sex, diagnosis, cytogenetics, white blood count, performance status, and Wheatley risk group were explored (supplemental Figure 2A-B). Although the numbers of patients in these analyses were small, there were no significant interactions between baseline variables and treatment of survival, and in particular no subgroup could be identified in which there was a benefit for vosaroxin either alone or in combination with LDAC. A similar lack of heterogeneity was found for FLT3-ITD and NPM1c mutations (data not shown).
Discussion
Even if older AML patients are candidates for intensive therapy, attempts to improve treatment have been disappointing.17-22 In addition, many patients have not been offered an intensive approach because they were considered unlikely to survive the rigors of treatment or, in some cases, because the priority for patients was quality rather than quantity of life. There is much less prognostic molecular information available in older patients and, because the overall results of therapy are poorer, such information has less prognostic impact.23 More extensive characterization of larger numbers of older patients may improve this situation. Attention to this population of patients was stimulated by our investigators, who realized that the therapeutic options on offer, which were exclusively a conventional chemotherapy approach, were not suitable for the “frail” patients who frequently presented (see the supplemental data for the investigators who recruited patients to the trial). This represents a major patient subgroup that historically has been offered palliation in the form of best supportive care. Initially, we tested whether regular LDAC given twice daily for 10 days was better than best supportive care.1 This trial was prematurely closed because of the significant superiority of the LDAC arm. There were no differences in recorded toxicities or supportive care requirements, so LDAC became our new standard of care for these patients. The benefit in this study was only observed in the 18% of patients who achieved CR, which led us to the conclusion that the achievement of remission was a useful surrogate for survival benefit. However, LDAC was far from satisfactory treatment, but it did serve as a standard against which to compare new treatments in this patient population. This led to the development of a rolling program of continuous randomized assessment of new options that we designated our Pick a Winner program in which new options are randomly compared with LDAC as a common control arm. This has been described in detail elsewhere.13
In the Pick a Winner program, since its initiation in Acute Myeloid Leukemia and High Risk Myelodysplastic Syndrome trial 16 to the point of data completeness on May 1, 2014, 1536 randomizations have been undertaken to evaluate 9 agents or combinations:19-22 evaluation is complete on 7 options, and 2 are ongoing. Mechanistically, vosaroxin has several properties that could be particularly relevant to older patients as outlined previously. In choosing a chemotherapeutic agent to test in this program, initial assessment is made of its characteristics and clinical activity. In particular, that the tolerability is appropriate. The phase 1 and 2 data available demonstrated that in patients with relapsed or refractory disease who were older than age 70 years, the 30-day mortality was low (7%)11,12 and toxicity was acceptable. We had also demonstrated in vitro that its efficacy was independent of P53 and that there was synergy with LDAC. We therefore initiated the 2 randomized comparisons that are reported here. Introduction of vosaroxin alone or in combination was introduced in a cautious manner in a limited number of experienced sites with enhanced site supervision. Disappointingly, neither monotherapy nor the combination met our criteria to continue the trial. In reaching their recommendation, the DMEC looked not only at the strict continuation criteria set down based upon remission but also relied upon safety data, and in particular early (60-day) mortality from both comparisons when deciding whether or not to continue. The DMEC closed the vosaroxin monotherapy arm based on a failure to improve the CR rate, but survival was also inferior. The combination arm satisfied the CR criteria for continuation, but the survival was worse, and there was evidence of increased 60-day mortality similar to the monotherapy arm. The confidence intervals of the HR for survival fell well short of suggesting a clinically relevant effect, so it was thought unlikely to become sufficiently superior with more patients or longer follow-up. A feature was the outcome of the LDAC arm. In these comparisons, the remission rates were 29% and 33% and the 12-month survivals were 31% and 37%. This reflects a general improvement in the outcome in the Pick a Winner program, in which there has been a variation in the LDAC arm result that does not appear to be explained by eligibility criteria. This endorses the need for randomization when assessing new agents in this patient population.
The difference between the arms in the combination comparison was attributable to an excess of deaths in the second month. If that is accounted for, there was no difference between either monotherapy or the combination. Patients who entered remission with vosaroxin did not have a more durable remission compared with LDAC, so a later survival benefit was thought to be unlikely. The excess deaths were attributed to the usual causes, and we found no suggestion of a treatment “center” effect in relation to treatment failures (P = .5 for heterogeneity between center and day 60 mortality in the vosaroxin combination arm), although the numbers from any 1 center were small.
For this group of patients, vosaroxin did not provide benefit. In spite of our careful “run-in” approach, the treatment was more intensive than anticipated. It is possible that it is beneficial alone or in combination in other circumstances such as relapsed/refractory disease in which the major international phase 3 Medtronic Vascular Talent Thoracic Stent Graft System for the Treatment of Thoracic Aortic Aneurysms trial (NCT01191801) is now completed24 or in high-risk patients as part of first-line therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sunesis Pharmaceuticals Inc. for supporting the trial, the United Kingdom Leukaemia and Lymphoma Research Fund for research support (LLR 13041), and the Haematology Clinical Trials Unit, Cardiff University, for managing the trial.
Authorship
Contribution: A.K.B. conceived the trial, developed the design, and wrote the manuscript; R.K.H. developed the trial design, analyzed the data, and wrote the manuscript; M.D., N.R., R.E.C., and D.M. coordinated the trial; C.H., N.P., M.-F.M., L.K., and H.D. recruited patients to the trial; I.F.T. coordinated data collection for the trial; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: A.K.B. served on an advisory board for Sunesis and R.K.H. acted on a data and safety monitoring board for Sunesis. Sunesis provided free drug and support for the trial’s drug shipment.
Correspondence: Alan K. Burnett, Department of Haematology, School of Medicine, Cardiff University, Heath Park, Cardiff CF4 14XN, United Kingdom; e-mail: akburnett719@gmail.com.