Key Points
This exploratory study describes the effect of eculizumab on multiple physiologic pathways affected by complement dysregulation in aHUS.
The results highlight the importance of sustained terminal complement blockade, even in patients with improved clinical laboratory values.
Abstract
Atypical hemolytic uremic syndrome (aHUS) is a genetic, life-threatening disease characterized by uncontrolled complement activation, systemic thrombotic microangiopathy (TMA), and vital organ damage. We evaluated the effect of terminal complement blockade with the anti-C5 monoclonal antibody eculizumab on biomarkers of cellular processes involved in TMA in patients with aHUS longitudinally, during up to 1 year of treatment, compared with in healthy volunteers. Biomarker levels were elevated at baseline in most patients, regardless of mutational status, plasma exchange/infusion use, platelet count, or lactate dehydrogenase or haptoglobin levels. Eculizumab reduced terminal complement activation (C5a and sC5b-9) and renal injury markers (clusterin, cystatin-C, β2-microglobulin, and liver fatty acid binding protein-1) to healthy volunteer levels and reduced inflammation (soluble tumor necrosis factor receptor-1), coagulation (prothrombin fragment F1+2 and d-dimer), and endothelial damage (thrombomodulin) markers to near-normal levels. Alternative pathway activation (Ba) and endothelial activation markers (soluble vascular cell adhesion molecule-1) decreased but remained elevated, reflecting ongoing complement activation in aHUS despite complete terminal complement blockade. These results highlight links between terminal complement activation and inflammation, endothelial damage, thrombosis, and renal injury and underscore ongoing risk for systemic TMA and progression to organ damage. Further research regarding underlying complement dysregulation is warranted. This trial was registered at www.clinicaltrials.gov as #NCT01194973.
Introduction
Atypical hemolytic uremic syndrome (aHUS) is a genetic, life-threatening disease of chronic, uncontrolled complement activation that leads to platelet, leukocyte, and endothelial cell activation, systemic thrombotic microangiopathy (TMA), and subsequent end organ damage or failure.1,2 aHUS is almost always caused by inherited or acquired defects in the activation of complement via the alternative pathway (AP),1 although rare mutations in DGKE and plasminogen have also been described.3,4 Historically, prognosis of patients with aHUS has been poor: up to 79% of patients die, require dialysis, or have permanent renal damage within 3 years of diagnosis.5
The role of the AP in the pathophysiology of aHUS is well described.1,6 The AP, an integral part of the innate immune response, is continuously active at low levels but amplified under conditions of infection or endothelial stress or damage.1 Under normal conditions, complement activation is tightly controlled by regulatory proteins, either soluble (eg, complement factors H or I) or membrane-bound (eg, decay accelerating factor [CD55], protectin [CD59], membrane cofactor protein [MCP; CD46], thrombomodulin [TM; CD141], and complement receptor 1 [CD35]).7 In aHUS, complement-associated gene mutations cause permanent loss of complement regulatory control. Gain-of-function mutations of genes encoding complement components and inhibitory autoantibodies directed at complement regulatory proteins can also lead to chronic AP overactivation.1
Devastating clinical effects observed in patients with aHUS likely result from the effect of uncontrolled complement activity on multiple physiologic pathways.8 C5a is postulated as a key link between inflammatory and thrombotic pathways9 and has potent proinflammatory effects.10 In addition, sublytic C5b-9 levels are associated with platelet, leukocyte, and endothelial activation and damage,11,12 all of which can further increase AP activation via a positive feedback loop.11,13,14
The kidney is particularly vulnerable to complement-mediated inflammatory injury occurring from deposition of circulating active complement fragments in the glomeruli and local (renal) complement production and activation.1,11 Increased C5b-9 deposits have been observed on glomerular endothelium and circulating platelets,11 both of which are affected in aHUS.1 The proximal tubule also has been identified as a focus of necrosis in aHUS-related TMA.15 The renal microvasculature is a common site for TMA, but aHUS may also lead to extrarenal manifestations in the central nervous, pulmonary, gastrointestinal, or cardiovascular systems in 50% or less of patients.16-19
The chronically activated complement has devastating clinical effects, and effective management of patients with aHUS through terminal complement blockade prevents TMA and end-organ damage.16,20 Eculizumab (Soliris; Alexion Pharmaceuticals, Inc., Cheshire, CT) is a humanized monoclonal antibody that blocks the cleavage of terminal complement protein C5 into the inflammatory C5a protein and C5b, a precursor of the lytic C5b-9 complex,21 and is the only approved treatment of aHUS.22,23 The efficacy and safety of eculizumab in aHUS was demonstrated in 2 prospective clinical trials.21 Subsequently, an open-label, single-group, multicenter, multinational, clinical trial (registered at www.clinicaltrials.gov as #NCT01194973) was conducted in adult patients with aHUS (C10-004).24 Inhibition of complement-mediated TMA with eculizumab led to rapid hematological improvements, significant improvements in renal outcomes, and discontinuation of plasma exchange/plasma infusion (PE/PI) and dialysis in most patients.24,25
The effect of terminal complement blockade with eculizumab on upstream AP activation or other markers of disease activity is unclear. We conducted a prespecified exploratory analysis as part of the C10-004 trial to investigate the effect of terminal complement blockade on biological markers associated with proximal and terminal complement activation,22,23,26 inflammation,27,28 endothelial cell activation and damage,28-35 coagulation,5,33,36,37 and renal injury38-40 in patients with aHUS.
Materials and methods
Clinical trial
This was a 26-week, open-label, nonrandomized, single-group, multicenter, trial24 of eculizumab in patients with aHUS in which patients could continue to receive eculizumab in an extension phase. Adult patients (≥18 years of age) with a diagnosis of aHUS were enrolled at 23 centers in North America and Europe. Eligible patients had platelet counts lower than 150 × 109/L, hemoglobin levels at or below the lower limit of the normal range, lactate dehydrogenase (LDH) levels 1.5 × upper limit of normal or more, serum creatinine levels at upper limit of normal or higher at screening, ADAMTS13 activity at 5% or higher, and no positive Shiga toxin-producing Escherichia coli test. An identified complement gene mutation, polymorphism, or factor H autoantibody was not required. Patients were vaccinated against Neisseria meningitidis and received appropriate antibiotics for 14 days if vaccination occurred less than 14 days before the first eculizumab dose. Eculizumab was administered intravenously at dosages of 900 mg once a week for 4 weeks, 1200 mg at week 5, and then 1200 mg every 2 weeks thereafter. The protocol was approved by the institutional review board at each participating center or by an independent ethics committee and was conducted in accordance with International Conference on Harmonisation Good Clinical Practice Guidelines and the Declaration of Helsinki. All patients provided written informed consent before study entry.
PE/PI
Patients were categorized according to whether or not they received PE/PI during the pretreatment period, defined as beginning on the start date of the current aHUS manifestation up to the first dose. The median PE/PI intensity was 0.5 sessions/day. Potential correlations between biomarker levels at baseline and the PE/PI rate/day or timing of the last PE/PI session during the pretreatment period were evaluated.
Biological samples
Serum, ethylenediaminetetraacetic acid plasma, and urine samples (N = 26-38, depending on sample matrix) were obtained from patients with aHUS at baseline, before eculizumab treatment, and at visits 3 (weeks 1-3), 6 (weeks 4-6), 12 (weeks 12-17), 17 (weeks 26-33), 18 (weeks 38-42), and 19 (weeks 49-54) during eculizumab treatment. For simplicity, visit 17 is referred to as week 26 and visit 19 as week 52 of treatment. Plasma samples were processed on ice as quickly as possible to preserve complement activity. Currently employed measures to prevent ex vivo complement activation, as well as to preserve complement, such as the addition of Futhan or use of BP100 tubes during blood acquisition, were not in place at the time this study was initiated, and therefore some complement assessments in plasma (C5a and sC5b-9) could not be reliably performed. C5a and sC5b-9 were reliably quantitated in urine, however. Freshly obtained urine was mixed (9:1) with 10 mM Tris buffer containing 0.05% Tween 20, 0.01% NaN3, and protease inhibitors (10 mM benzamidine, 10 mM ε-aminocaproic acid, 20 mM ethylenediaminetetraacetic acid, and 100 U/mL aprotinin) before centrifugation at 4°C. All samples were aliquoted and stored at −80°C until analysis, without subsequent freeze-thaw. Samples were not available from every patient at every point, but all available samples at each point were evaluated. Samples from patients who discontinued eculizumab therapy were not available.
Serum samples from clotted whole blood from 20 healthy volunteers (HV; 10 men, 10 women) were purchased (BioreclamationIVT, Westbury, NY). Ten plasma samples (4 women, 6 men) and 10 urine samples (8 women, 2 men) were freshly obtained from HV selected randomly from a donor pool, according to institutional guidelines. All donors were between the ages of 18 and 68 years. Pregnant donors or those with recent surgery or illness were excluded. Plasma and urine samples from HV were processed and stored under similar conditions as described earlier for patient samples.
Biomarker detection
Levels of plasma and serum markers were evaluated from HV and patients with aHUS at baseline and at regular intervals during eculizumab treatment over the course of a 1-year period; markers in urine were evaluated over the course of 26 weeks of eculizumab treatment. Detailed methods for measurement of levels of plasma complement Ba, serum soluble tumor necrosis factor receptor-1 (sTNFR1), plasma prothrombin fragment 1+ 2 (F1+2), plasma TM, urinary cystatin C, tissue inhibitor of metalloproteinases-1 (TIMP-1), β2-microglobulin (β2-M), liver fatty acid binding protein (L-FABP-1), creatinine, sC5b-9, and C5a are described in the supplemental Methods, available on the Blood Web site.
Mutation/polymorphism analysis
Patient samples were categorized according to the absence (no identified mutation) or presence (identified mutation) of complement gene mutations/polymorphisms detailed from the medical record during screening. Mutational status of patients who enrolled in the study with no previously identified complement gene mutations/polymorphisms was confirmed by central laboratories (University of Iowa and Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou) and included whole-gene sequence analyses of the CFH, CFI, CD46 (MCP), CFB, and C3 genes, including exon–intron junctions and multiplex ligation-dependent probe amplification analyses to detect deletions or duplications of the CFHR3-CFHR1 region.
Statistical analysis
Patient biomarker levels at baseline, week 26, and week 52 were compared with HV biomarker levels using Wilcoxon rank sum test. Baseline biomarker levels among HV and patients receiving PE/PI of varying intensities were compared using the Kruskal-Wallis test. The effect of PE/PI on baseline biomarker levels was also evaluated by linear regression analysis, evaluating potential correlations between either the PE/PI intensity rate or the day of last PE/PI session relative to baseline biomarker levels measured on day 0. Changes in biomarker levels with ongoing eculizumab treatment are described using box-whisker graphs showing median, 25th and 75th percentiles, and range. Change from baseline in biomarker levels at each postdose point were statistically analyzed using a restricted maximum likelihood-based, repeated measures approach (mixed-effect analysis of variance model) among patients with elevated biomarkers at baseline.
Results
Patients
Forty-one adult patients with aHUS were treated; 38 (93%) completed the initial 26-week clinical study period and 21 (51%) continued treatment of 1 year during the optional extension period. Twenty patients (49%) had 1 or more identified complement gene mutation and/or factor H autoantibody. Thirty patients (73%) enrolled during their first identified clinical TMA manifestation. At screening, mean ± standard deviation platelet count was 119.1 ± 66.1 × 109/L, mean haptoglobin was 0.6 ± 0.40 g/L, and mean LDH level was 492.9 ± 500.9 U/L. Thirty-five patients (85%) received PE/PI before eculizumab. Patients who received PE/PI averaged 9.6 sessions (range, 1-26 sessions) during the pretreatment period, with a median rate of 0.5 sessions/day. Twenty-four patients (59%) were receiving dialysis at baseline, and 9 (22%) had a history of prior renal transplantation. Thirty-three patients (80%) had chronic kidney disease stage 4 or 5 (ie, estimated glomerular filtration rate <30 mL/minute/1.73 m2). Additional patient demographics and disease characteristics were described previously.24 Adult HV from whom control serum, plasma, and urine were obtained are described in “Materials and methods”.
Biomarker levels at baseline
Evaluated markers of proximal and terminal complement activation, inflammation, endothelial cell activation and damage, and coagulation and renal injury are detailed in Table 1.10,12,13,22,23,26-32,36-61 At baseline, all markers were significantly elevated in the majority of patients with aHUS compared with levels measured in adult HV (Table 2). Plasma Ba levels were elevated in all (35/35) patients at baseline, with median levels 6-fold higher than levels in HV, indicating systemic AP activation. Terminal complement activation markers (urine [U-]C5a and U-sC5b-9) were elevated by 45- and 305-fold, respectively, in more than 85% of patients with aHUS, and only 1 patient did not demonstrate elevated C5a or sC5b-9. All patients showed elevated serum sTNFR1 levels at baseline (19-fold higher than levels observed in HV), demonstrating evidence of systemic inflammation. Soluble vascular cell adhesion molecule-1 (sVCAM-1) and plasma TM levels were elevated by 2- and 4-fold in more than 94% of patients, reflecting endothelial activation and injury, respectively. Baseline plasma F1+2 and d-dimer levels were also 6- and 10-fold higher than levels observed in HV in more than 94% of patients, indicating thrombin generation and fibrinolysis (Table 2). At baseline, 69% to 83% of patients with aHUS also showed significantly elevated levels of candidate renal injury biomarkers associated with acute kidney injury, ischemic injury, and nephrotoxicity in rodent models and human disease, including those associated with proximal tubular injury (U-cystatin-C,38,53-55 clusterin,38,58,59 and β2-M38,60 ), interstitial injury (TIMP-140,56 ), and deteriorating renal function (U-L-FABP-139,57 ); levels of these markers were 9- to 48-fold higher than levels measured in HV (Table 2).
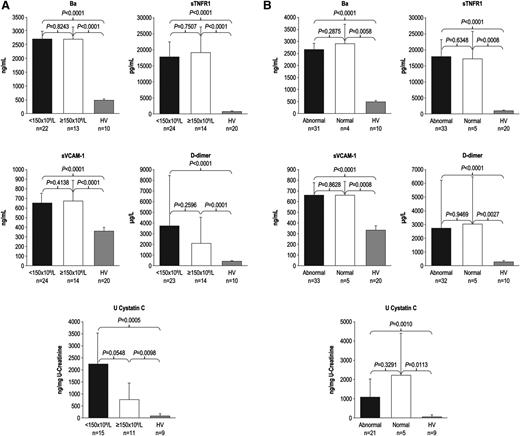
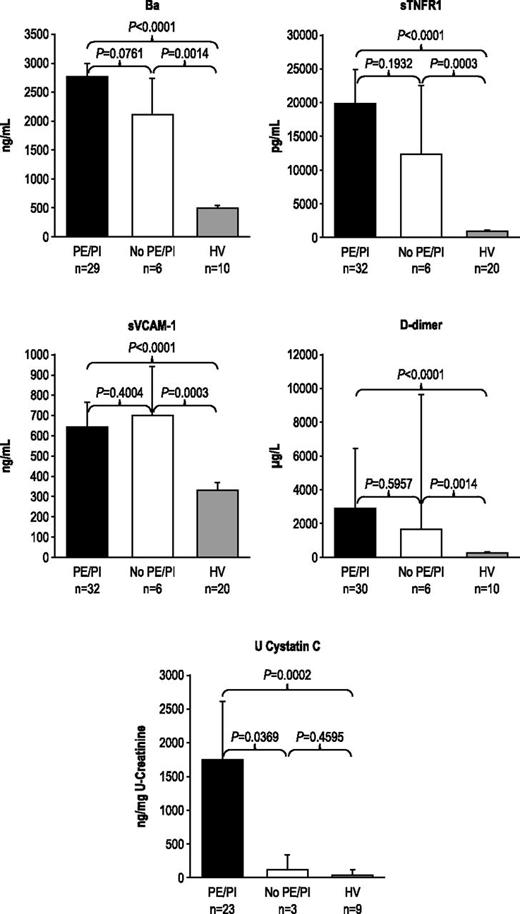
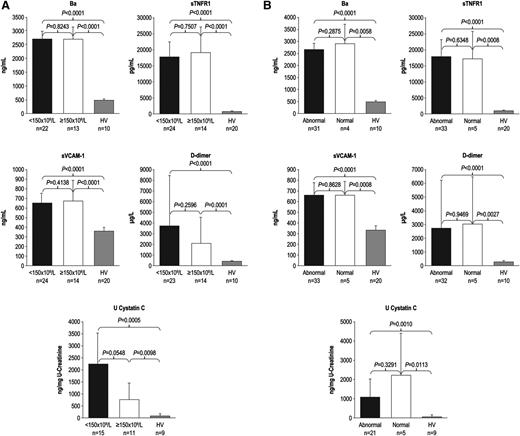
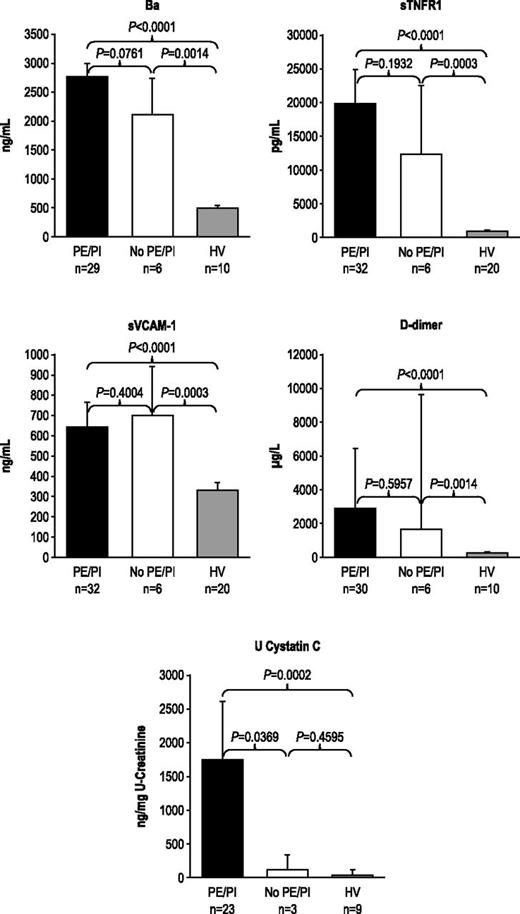
During the 7-day period from screening/enrollment to baseline, platelet, LDH, or haptoglobin levels returned to normal values in some patients, allowing evaluation of markers among patients with normal baseline hematologic or biochemical laboratory values. Patients with normal platelet count, LDH, or haptoglobin levels at baseline demonstrated elevated levels of Ba, sTNFR1, sVCAM-1, d-dimer, U-cystatin C (Figure 1A-B), and all other markers (U-C5a, U-sC5b-9, F1+2, TM, U-clusterin, U-TIMP-1, U-β2-M, U-L-FABP-1 not shown). In addition, patients receiving repeated PE/PI before eculizumab treatment also showed elevated levels of every marker evaluated (Figure 2; U-C5a, U-sC5b-9, F1+2, TM, U-clusterin, U-TIMP-1, U-β2-M, U-L-FABP-1 not shown). Patients showed elevated biomarker levels at baseline regardless of less frequent (≤0.5 sessions/day) or more frequent PE/PI (>0.5 sessions/day) (P < .0027 for all compared with HV; see supplemental Figure), and linear regression analysis confirmed that intensity of pretreatment PE/PI was not correlated with biomarker levels at baseline (correlation coefficient = −0.0873 to 0.3218; P ≥ .10 for all). Although the timing of baseline blood collection relative to the last PE/PI session was variable, there was no correlation between timing of the last PE/PI session and baseline biomarker levels (correlation coefficient = −0.0094 to 0.3611; P ≥ .0830 for all).
Biomarker levels in patients with normal platelet counts and normal LDH and haptoglobin levels at baseline. Median levels of markers of complement activation, vascular inflammation/damage and coagulation, and renal injury were elevated in patients with aHUS with and without (A) normal platelet count and (B) normal LDH and haptoglobin levels at baseline. Error bars represent 95% confidence intervals.
Biomarker levels in patients with normal platelet counts and normal LDH and haptoglobin levels at baseline. Median levels of markers of complement activation, vascular inflammation/damage and coagulation, and renal injury were elevated in patients with aHUS with and without (A) normal platelet count and (B) normal LDH and haptoglobin levels at baseline. Error bars represent 95% confidence intervals.
Biomarker levels in patients receiving PE/PI at baseline. PE/PI does not affect biomarkers of complement activation, vascular inflammation/damage and coagulation, and renal injury in patients with aHUS compared with HV at baseline. Median levels and 95% confidence intervals are represented.
Biomarker levels in patients receiving PE/PI at baseline. PE/PI does not affect biomarkers of complement activation, vascular inflammation/damage and coagulation, and renal injury in patients with aHUS compared with HV at baseline. Median levels and 95% confidence intervals are represented.
Biomarker levels during eculizumab treatment
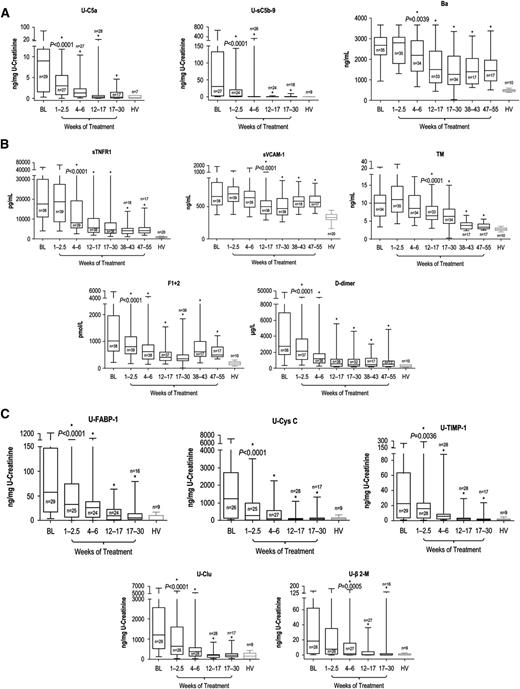
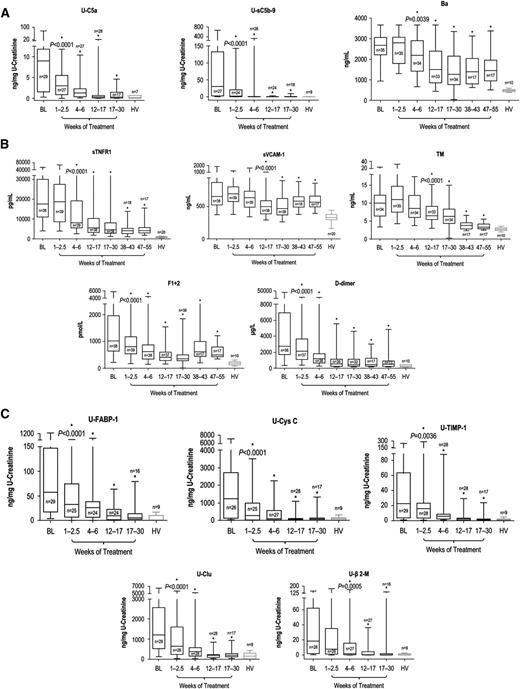
All biomarkers described were reduced with eculizumab. Blockade of terminal complement activation21 led to sustained reductions in levels of markers of complement activation (Figure 3A). Patients demonstrated immediate and sustained reductions in U-C5a and U-sC5b-9 after the first dose of eculizumab (P < .0001 vs baseline by 2.5 weeks and at all later points) to levels consistent with those of HV (P = .3694 and P = .2552, respectively, vs HV, after the first eculizumab dose), with up to 100% reduction observed at week 26 (mean percentage reduction of 90% ± 7.48% and 98% ± 1.39%, respectively). The rapid decrease in terminal complement activation was followed by a reduction at week 6 in plasma Ba levels (Figure 3A; P = .0039 vs baseline levels) and at all later points (P ≤ .0001 for all) (mean percentage reduction of 30% ± 14.05% at week 52). Ba remained significantly elevated compared with levels in HV (3-fold versus HV at week 52; P ≤ .0001).
Biomarker levels during terminal complement blockade with eculizumab. Longitudinal decreases in median levels of biomarkers of (A) complement activation, (B) vascular inflammation/damage and coagulation, and (C) renal injury were demonstrated with eculizumab therapy in patients with aHUS compared with HV. Changes in biomarker levels with ongoing eculizumab treatment are displayed using box-whisker graphs showing median, 25th and 75th percentiles, and range. *Levels were significantly reduced compared with baseline; the P value of reduction at the first significant time point is shown.
Biomarker levels during terminal complement blockade with eculizumab. Longitudinal decreases in median levels of biomarkers of (A) complement activation, (B) vascular inflammation/damage and coagulation, and (C) renal injury were demonstrated with eculizumab therapy in patients with aHUS compared with HV. Changes in biomarker levels with ongoing eculizumab treatment are displayed using box-whisker graphs showing median, 25th and 75th percentiles, and range. *Levels were significantly reduced compared with baseline; the P value of reduction at the first significant time point is shown.
Ongoing treatment with eculizumab also led to reduced levels of biomarkers of inflammation, endothelial activation and damage, and coagulation (Figure 3B). sTNFR1 levels were significantly reduced from baseline levels by week 6 and at all later points (P < .0001 vs baseline). By week 52, sTNFR1 levels decreased by up to 94% (mean percentage reduction of 60% ± 14.27%) but remained elevated (5-fold; P < .0001 compared with HV). Median sVCAM-1 and TM levels were significantly decreased from baseline by week 17 (P < .0001 for both), and reduction was sustained at all later points (P ≤ .005). Although median sVCAM-1 levels remained elevated (1.7-fold compared with HV [P < .0001]) at week 52, TM levels decreased by up to 77% (mean percentage reduction of 60% ± 10.32%) to levels near those of HV (1.2-fold higher than HV; P = .022) at that point. With eculizumab, median F1+2 and d-dimer levels both decreased by week 2.5 (P < .0001) and at all points thereafter (P ≤ .05). F1+2 levels decreased by up to 89% (mean percentage reduction of 26% ± 27.39%) by week 52, and d-dimer levels decreased by up to 99% (mean percentage reduction of 47% ± 58.34%). At week 52, levels of both coagulation markers remained modestly elevated (P ≤ .007 for both) at 2.6- and 1.8-fold above the levels observed in HV, respectively.
Eculizumab treatment significantly reduced levels of all renal injury markers by week 6; reduction was sustained at all later points (P ≤ .0005 for all), and markers were decreased to levels consistent with HV (P > .1309 for all) by week 26, with the exception of U-TIMP-1, which remained 2.5-fold higher (P = .0188; Figure 3C).
Analysis by complement genetic mutation/polymorphism
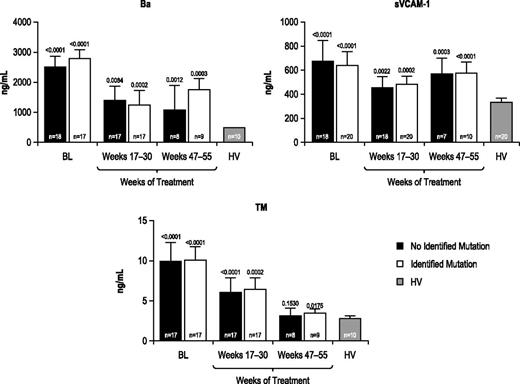
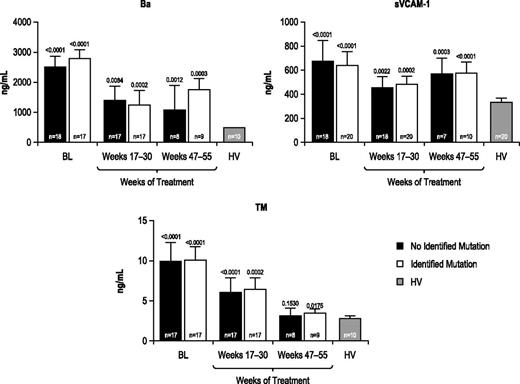
Marker levels were evaluated before and during eculizumab treatment among patients with and without identified complement regulatory gene mutations/polymorphisms. All markers (Table 2) were elevated at baseline relative to those observed in HV, regardless of the presence or absence of an identified complement gene mutation, and all markers were reduced during 1 year of treatment with eculizumab (representative results for Ba, sVCAM-1, and TM shown in Figure 4).
Biomarker levels in patients with or without an identified complement regulatory gene mutation or polymorphism. Biomarker profiles were elevated at baseline and were reduced during eculizumab treatment in patients with aHUS with or without an identified complement regulatory gene mutation/polymorphism compared with HV. Longitudinal median biomarker levels are shown with P values relative to HV levels indicated.
Biomarker levels in patients with or without an identified complement regulatory gene mutation or polymorphism. Biomarker profiles were elevated at baseline and were reduced during eculizumab treatment in patients with aHUS with or without an identified complement regulatory gene mutation/polymorphism compared with HV. Longitudinal median biomarker levels are shown with P values relative to HV levels indicated.
Discussion
Results of this exploratory analysis of the C10-004 trial more fully characterize the physiologic pathways affected by complement dysregulation and provide insight into the effect of eculizumab on these pathways in aHUS. At baseline, patients had significantly elevated levels of markers associated with proximal and terminal complement activation, systemic inflammation, endothelial cell activation and damage, coagulation, and renal injury. Elevated marker levels at baseline were observed in the majority of patients, even in patients with normal baseline platelet, LDH, or haptoglobin levels, and regardless of prior use of PE/PI or mutation status.
All patients presented with significant proximal AP activation, as evidenced by elevated Ba, confirming the fundamental complement-mediated pathophysiology of aHUS. The continued elevation of Ba after 1 year of eculizumab treatment demonstrates that unregulated AP activation in patients with aHUS is persistent, despite complete inhibition of terminal complement activity by eculizumab. This finding is not unexpected, as AP activation occurs upstream of C51 and would be expected to continue even with downstream, complete terminal complement C5 blockade.21 Consistent with baseline AP activation, patients with aHUS also demonstrated significant terminal complement activation at baseline, as high levels of both urine C5a and sC5b-9 were observed for most patients, including those with no identified complement gene mutations. Although a potential study limitation is the absence of plasma C5a and sC5b-9 level measurements, urine sampling to determine terminal complement activation is well established. Furthermore, urine levels may better reflect local complement activation; indeed, urinary sC5b-9 levels have been shown to correlate highly with increased tubulointerstitial C3 synthesis,62 local deposition in the glomerular and tubular epithelium, and disease severity in patients with membranous nephropathy.63 In our study, both urine C5a and sC5b-9 levels decreased immediately after the first eculizumab dose, quickly reaching levels observed in HV. Our results in this large cohort of patients with aHUS contrast with those of a recent single case report in which eculizumab therapy resolved HELLP syndrome (hemolysis, elevated liver enzymes, low platelets), decreased both plasma and urine sC5b-9 levels, and reduced complement hemolytic activity, but did not appear to reduce plasma C5a/C5a(desArg) levels.64 Additional studies may reveal whether these disparate results are caused by differences in assay methodology or reagent specificity; many, if not all, C5a/C5a(desArg) antibodies cross react with C5 (and potentially with C5 bound to eculizumab).
Elevated baseline sTNFR1 levels also were observed in all patients, highlighting the systemic inflammation present in aHUS. sTNFR1 levels were reduced significantly by week 6, and profoundly within 1 year of treatment with eculizumab. These results suggest that elevated terminal complement activation in aHUS is a major contributor to inflammation, shown to play a central role in the pathophysiology of both acute kidney injury65,66 and progressive renal disease.67 Interestingly, although Ba levels remained elevated during 1 year of treatment, median levels were decreased by 30%, suggesting that C5 blockade with eculizumab may mitigate upstream AP activation. Potential mechanisms are suggested by data showing that sublytic C5b-9 induces multiple effects on endothelial cells, including apoptosis, cellular retraction, and exposure of extracellular matrix,13,68 which in turn may activate the alternative pathway directly.13,69
Inflammation in aHUS also has been shown to contribute to endothelial cell activation and damage, evidenced by significant elevations in baseline sVCAM-1 and TM levels, respectively.11 After 1 year of treatment, sVCAM-1 remained elevated relative to HV levels, whereas TM was reduced to near-normal levels. Importantly, levels of markers associated with renal injury were reduced and became indistinguishable from levels in HV by 26 weeks. These biomarker results are consistent with the renal impairment and improvement observed in the trial and indicate that terminal complement blockade with eculizumab interrupts progression from inflammation to renal injury and organ dysfunction, despite evidence of persistent AP and endothelial cell activation.70
Virtually all patients had elevated F1+2 and d-dimer levels at baseline, indicating thrombin generation and fibrinolysis. Increased F1+2 levels preceded renal injury in other TMA studies in Shiga toxin-producing Escherichia coli HUS.71,72 Reduction of F1+2 and d-dimer levels with eculizumab suggests that prevention of C5a and C5b-9 formation and bioactivity, through terminal complement blockade, downregulates the coagulation cascade. This is consistent with a study of paroxysmal nocturnal hemoglobinuria, which demonstrated that eculizumab treatment results in a significant decrease in thrombosis73 and in markers associated with thrombin generation and reactive fibrinolysis, including plasma d-dimer, thrombin-anti-thrombin complexes, interleukin 6, soluble P-selectin, and microparticles with prothrombotic activity.33 Reduction in F1+2 and d-dimer with continued terminal complement blockade in patients with aHUS is also consistent with clinical findings from this study demonstrating rapid and complete inhibition of TMA after eculizumab treatment initiation.25
In patients with aHUS, both with and without identified complement mutations, markers were significantly elevated at baseline, and ongoing eculizumab therapy significantly reduced levels in both subsets of patients. A recent retrospective study among a subset of patients enrolled in an aHUS/thrombotic thrombocytopenic purpura registry that fulfilled the diagnostic criteria for aHUS reported elevated levels of Bb, plasma C5a, and sC5b-9 among patients with and without complement regulatory gene mutations.74 Although differences in methodology and matrices evaluated preclude a direct comparison of biomarker levels, similar trends were observed, and together, these results confirm recent recommendations that an identified complement gene mutation is not required for aHUS diagnosis or initiation of eculizumab therapy.16,20
It should be stressed that the biomarkers characterized in our study have not yet been validated to confirm aHUS diagnosis or to make other clinical decisions in dosing or treatment. Indeed, there is disagreement on the utility of using individual markers to monitor disease activity in aHUS, and no single marker has been validated for this purpose. Although some have described using CH50 assays to monitor patients receiving eculizumab,75-77 others have shown the insensitivity of CH50 or plasma C5a and sC5b-9 assays to fully assess ongoing aHUS disease activity compared with assays that reflect endothelial complement deposition.78 Regulatory guidance includes reference to potential risk for TMA manifestations after eculizumab discontinuation.22,23 Recent evidence from case studies79-84 and an observational study85 suggest that although certain individual patients discontinued therapy without safety concerns, 6 (38%) of 16 patients had TMA manifestations after eculizumab discontinuation.
It is remarkable that even patients with normal baseline laboratory values for platelet count, LDH, and haptoglobin or use of PE/PI before eculizumab had strong evidence of complement activation, vascular inflammation, coagulation, endothelial activation, and damage and renal injury. Other studies also have shown that C3 consumption and complement deposition on platelets and endothelium occur during periods when a clinical manifestation is not evident.78,86 The current study extends these observations, demonstrating that underlying complement dysregulation continues even with PE/PI management and in the absence of overt symptoms.
Taken together, these results point to associations between biomarkers and disease progression and organ damage in aHUS: chronic AP activation leads to C5a and C5b-9 formation and TNF-α elaboration,28,51 which directly activates and damages endothelial cell surfaces, inducing VCAM-1 expression29,30 and TM shedding.31,32 C5a-driven VCAM-1 expression is also increased,41 whereas C5b-9 deposition on endothelial cell surfaces further induces endothelial activation and damage,12,43,45 provoking additional AP activation, which is improperly regulated.13 Thus, chronic AP overactivation and ensuing elaboration of potent inflammatory mediators leads to increased endothelial cell activation, injury, and thrombosis, resulting in the clinical phenotype of TMA and progression to extrarenal injury and end-stage renal disease.
Ongoing terminal complement inhibition with eculizumab treatment markedly reduces inflammation and coagulation and decreases endothelial activation and damage. Although significantly reduced during 1 year of treatment with eculizumab, persistently elevated Ba and sVCAM-1 levels in patients with aHUS reflect the effect of genetic dysregulation of AP and ongoing activation upstream of C5 and the subsequent propensity for endothelial cell activation. By blocking terminal complement activity, eculizumab reduces ongoing endothelial damage and renal injury, even in the presence of chronic AP activation. Although limited by low numbers of patients, these data underscore the ongoing risk for systemic TMA and progression to organ damage and highlight the need for further research into underlying complement dysregulation in patients with aHUS with or without clinical TMA manifestations.
Aspects of these data were presented at the 55th American Society of Hematology Annual Meeting and Exposition, New Orleans, LA, December 8, 2013; the 51st European Renal Association–European Dialysis and Transplant Association Congress, Amsterdam, The Netherlands, May 31-June 3, 2014; and the Society of Nephrology Kidney Week 2014 annual meeting, Philadelphia, PA, November 11-16, 2014.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Kristen W. Quinn, PhD, of Peloton Advantage, LLC, and John F. Kincaid, MD, MA, and Erin Harvey, MSc, of Alexion Pharmaceuticals, Inc., for assistance with manuscript preparation; Rong Lin, MD, MPH, Jimmy Wang, PhD, Joseph Yen, PhD, and Marcia Wang, PhD, for biostatistical analyses; and the Alexion Pharmaceuticals clinical data management and operations teams for study operations and data integrity. We are deeply indebted to the patients, patients’ families, and clinicians who participated in the C10-004 study. This study was sponsored by Alexion Pharmaceuticals, Inc.
Authorship
Contribution: R.C., A.K., C.L.B., and S.J.F. were responsible for study design, data analysis, and interpretation and wrote the manuscript; R.C., A.K., and K.B. designed biomarker panels, validated the assays, and performed the research; S.J.F. and C.L.B. directed the project, interpreted the data, and wrote the manuscript; M.O. and C.L.B. provided essential input on patient data; and Y.Y. and A.P.M. collected, processed, and organized patient and control samples and assisted with data organization and review. All authors have reviewed and approved the manuscript.
Conflict-of-interest disclosure: All authors are employees and stock shareholders of Alexion Pharmaceuticals, Inc.
Krystin Bedard died on January 12, 2014.
Correspondence: Susan J. Faas, Alexion Pharmaceuticals, Inc., 352 Knotter Dr, Cheshire, CT 06410; e-mail: FaasS@alxn.com.