Key Points
Pregnant women with sickle cell disease have high risks of adverse maternal and perinatal outcomes.
The risks are greatest for those with HbSS disease (vs HbSC disease) and those in low income (vs high income) countries.
Abstract
A systematic review and meta-analysis of observational studies were conducted to quantify the association between sickle cell disease in pregnancy and adverse maternal and perinatal outcomes. Data sources (Medline, Embase, Maternity and Infant care, Cochrane, Web of Science, Popline) were searched for publications to June 2014. Eligibility criteria included observational studies reporting maternal and perinatal health outcomes in pregnant women with sickle cell disease against a comparative group of pregnant women without sickle cell disease. Twenty-one studies (including 26 349 women with sickle cell disease; 26 151 746 women without sickle cell disease) were eligible for inclusion. Pregnancies in women with HbSS genotype, compared with women without sickle cell disease, were at increased risk of maternal mortality (relative risk [RR], 5.98; 95% confidence interval [CI], 1.94-18.44), preeclampsia (RR, 2.43; 95% CI, 1.75-3.39), stillbirth (RR, 3.94; 95% CI, 2.60-5.96), preterm delivery (RR, 2.21; 95% CI, 1.47-3.31), and small for gestational age infants (RR, 3.72; 95% CI, 2.32-5.98). Meta-regression demonstrated that genotype (HbSS vs HbSC), low gross national income, and high study quality were associated with increased RRs. Despite advances in the management of sickle cell disease, obstetrics, and neonatal medicine, pregnancies complicated by the disease remain associated with increased risk of adverse maternal and perinatal outcomes.
Introduction
Sickle cell disease (SCD) is defined as an autosomal recessive hemoglobinopathy that includes sickle cell HbSS disease and various compound heterozygous genotypes (eg, sickle cell HbSC disease or sickle cell β-thalassemia disease [HbSβ thal]) characterized by chronic hemolytic anemia and vaso-occlusive complications.1 It is one of the most common genetic disorders globally, with >300 000 children born with the condition each year.2,3 The disease is associated with high lifetime morbidity and premature mortality,4 as described in the most recent Global Burden of Disease study.5 Better quality of care for individuals with SCD has improved survival, and thus there are increasing numbers of women reaching reproductive age.6
Individual studies have shown that pregnant women with SCD are at increased risk of maternal and fetal adverse outcomes; however, the heterogeneity inherent in the pathophysiology of SCD and in the setting of these studies leads to uncertainty when estimating the risk associated with pregnancy for women with the disease.7 The lack of comprehensive data on pregnancy outcomes in these women has made provision of antenatal counseling, clinical care pathways, and benchmarks for health care in institutions worldwide challenging. The aim of this study was to systematically review and meta-analyze maternal and perinatal outcomes in pregnant women with SCD, with consideration of factors that may influence heterogeneity between studies.
Methods
Search strategy
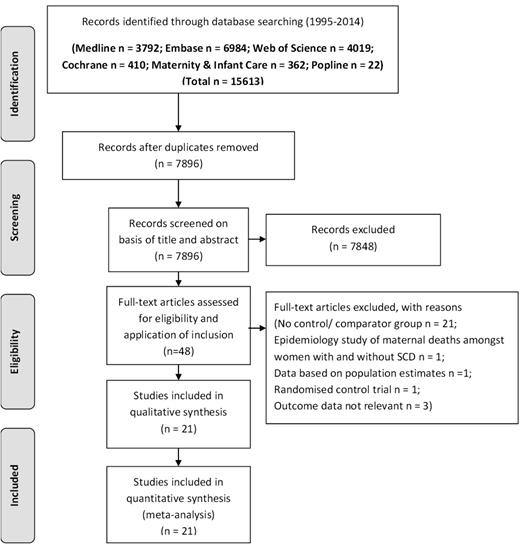
A systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and Meta-Analysis of Observational Studies in Epidemiology Group criteria.8 The following data sources were interrogated: MEDLINE, EMBASE, Cochrane, Popline, Web of Science, and Maternity and Infant Care. Where the option existed, literature searches were restricted to between January 1995 and June 1, 2014 publication. The search terms are shown in Table 1. Abstracts and full text articles were inspected by 2 authors (E.O.-N., D.M.) to identify those articles that met inclusion criteria (Figure 1). In addition, we interrogated references from relevant original papers and review articles to identify further relevant studies. There were no language restrictions.
Study selection
Studies were included in the systematic review if they met the following criteria: the study design was observational; the exposure of interest was SCD in pregnancy (stratified into SS disease, SC disease, and those reported as SCD but with the genotype not specified); maternal and/ or perinatal outcomes were included; and the investigators reported absolute numbers or relative risks with 95% confidence intervals. We excluded reviews, editorials, studies without a comparator group (with no SCD), nonhuman studies, and letters without sufficient data. Studies where women with a sickle cell trait were included as cases were also excluded. If study populations were reported more than once across several papers, we used the dataset with the longest follow-up period to avoid duplication.
Data extraction
Data extraction was carried out independently by 2 authors (D.M. and L.W.) using a standard extraction form. The following information from each study was extracted: authors; year of publication; study name; study design; study location; number of women in exposed and comparator groups; genotype; and outcomes. For each included study, data were retrieved for the meta-analysis on the outcomes of interest: maternal mortality, preeclampsia, eclampsia, caesarean delivery, stillbirth, neonatal death, preterm delivery (delivery before 37 completed weeks of gestation), and delivery of a small for gestational age infant (<10th centile). Details on use of blood transfusion (routine/for clinical indications and top-up/exchange) were collected for each study. We obtained the gross national income per capita for each study location from World Bank data.
Quality assessment
Quality assessment was performed using the Newcastle-Ottawa (NOS) scale.9 Studies were judged on 3 broad perspectives, with a maximum of 9 stars available: the selection of the study groups (4 stars allocated to selection based on a representativeness of the exposed, selection of the nonexposed, ascertainment of the exposed, and demonstration that the outcome of interest was not present at the start of the study); comparability of the groups (2 stars allocated to comparability of the groups in terms of appropriate study comparison group); and ascertainment of either the exposure or the outcome of interest for case-control or cohort studies, respectively (3 stars allocated for assessment of outcome, length of follow-up, and adequacy of follow-up). The papers were categorized into high- (7-9), medium- (4-6), and low-grade (1-3) quality.
Statistical analysis
Analyses were pregnancy based. The main measure of effect of maternal SCD on maternal and pregnancy outcome was the unadjusted risk ratio, calculated from the given numbers of pregnancies and events. Separate comparisons were made for women with HbSS, HbSC, and genotype not specified. In each analysis, the reference group was women without SCD. A small proportion of women contributed >1 pregnancy to a study, but without individual patient data, it was not possible to adjust the confidence intervals for the relatively small amount of clustering caused by including >1 pregnancy per woman.
Depending on the outcome under consideration, studies with no events in either arm were excluded. We used statistical meta-analysis with a random effects model10 to assess the association between SCD and adverse pregnancy outcome. The level of heterogeneity is expressed using τ2 (rather than I2),11 a measure of the absolute level of unexplained variation between the studies. As with I2, a τ2 value of 0 indicates no unexplained variation, whereas values of τ2 ∼0.5 indicate a typical ratio of 2 between study estimates of the risk ratio; higher values indicate higher levels of variation. Unlike I2, there is no artificial upper limit of 100%.
We also conducted analysis stratified by sickle cell genotype (as specified above) and high vs low/medium resources health care, as well as quality of study based on NOS. Where substantial heterogeneity existed (by τ2), multilevel mixed-effects logistic regression10 was used to test for study differences using the following variables: genotype (SC vs SS and SCD genotype not specified vs SS); gross national income (GNI) per capita; and NOS. The results are expressed as odds ratios.
All statistical analyses were performed in the statistical package Stata, version 12.1 (StataCorp, College Station, TX) using the commands metan and xtmelogit.11
Results
A flow diagram for identification of papers and subsequent evaluation is shown in Figure 1; after excluding ineligible papers (as shown), 21 papers remained.
Study characteristics
The characteristics of the 21 studies included in the meta-analysis are outlined in Table 2 (HbSS disease12-23 ), Table 3 (HbSC disease6,17,18,20,23 ), and Table 4 (SCD with genotype not specified).24-31 All studies were categorized as pregnancy cohort studies. Thirteen studies originated from high income countries, with the remaining 8 studies from medium/low income countries. Five studies were considered to be of high quality, 15 of medium quality, and 1 of low quality (Table 5). The meta-analysis included 26 349 women (26 823 pregnancies) with SCD in pregnancy, including 1276 women (1523 pregnancies) in women with HbSS disease, 279 women (331 pregnancies) in women with HbSC disease, and 24 794 (24 969 pregnancies) in women with SCD with genotype not specified, together with 26 151 746 women without SCD or women from the general population assumed not to have SCD (26 185 638 pregnancies). It was estimated that 1.8% of women contributed >1 pregnancy to the total pregnancy numbers. Of the 21 studies, 10 stated that the comparator population was matched for ethnicity; of the remaining 11 studies, 10 were from countries with a relatively low degree of diversity such that women in the control group were likely to be comparable to the women with SCD.
Association between SCD and maternal outcome
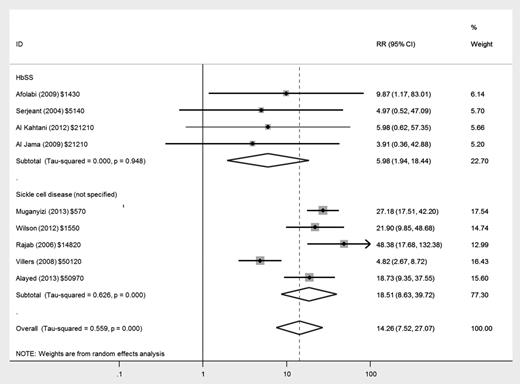
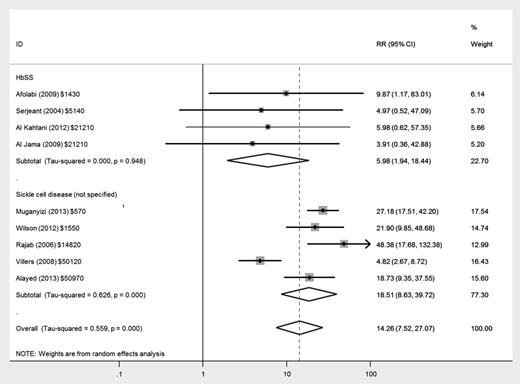
There was an increased risk of maternal mortality in women with HbSS disease (relative risk [RR], 5.98; 95% confidence interval [CI], 1.94-18.44) and in women with nonspecified SCD (RR, 18.51; 95% CI, 8.63-39.72) compared with women without SCD, but there was much greater heterogeneity between studies in the latter group (Figure 2). There was only 1 death (of 279 women in total) in studies reporting women with HbSC disease as a separate group.17
Forest plot of studies investigating the association of SCD with maternal mortality.
Forest plot of studies investigating the association of SCD with maternal mortality.
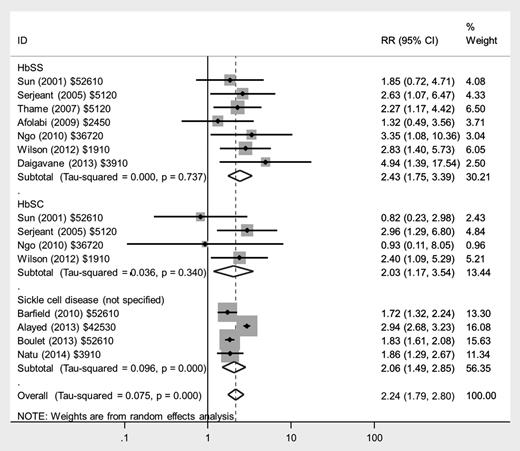
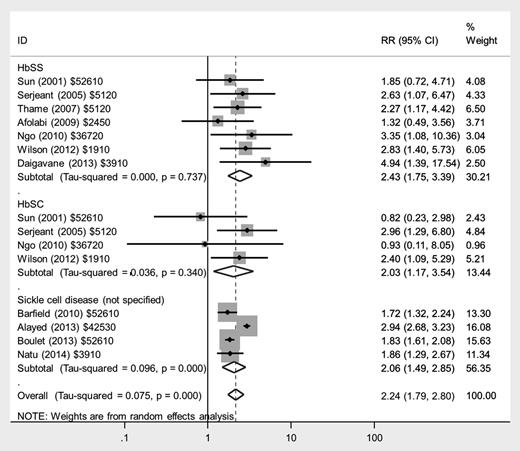
There was an approximate doubling in the risk of preeclampsia in women with HbSS disease (RR, 2.43; 95% CI, 1.75-3.39), HbSC disease (RR, 2.03; 95% CI, 1.17-3.54), and those with nonspecified SCD (RR, 2.06; 95% CI, 1.49-2.85) compared with women without SCD (Figure 3), with low heterogeneity between studies. There was a similarly increased risk of eclampsia in women with HbSS disease (RR, 4.89; 95% CI, 1.97-12.16), but no significantly increased risk in women with HbSC disease or nonspecified SCD compared with women without SCD.
Forest plot of studies investigating the association of SCD with preeclampsia.
Women with HbSS disease had a small increase in risk of being delivered by Caesarean section (RR, 1.50; 95% CI, 1.26-1.79), as did those with HbSC disease (RR, 1.56; 95% CI, 1.31-1.87) and nonspecified SCD (RR, 1.27; 95% CI, 1.18-1.36) compared with those without SCD.
Association between SCD and adverse perinatal outcome.
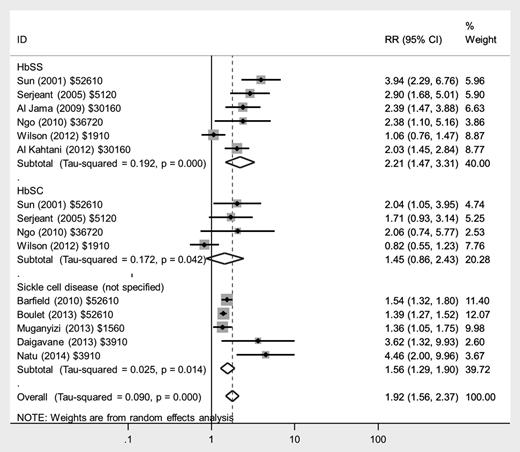
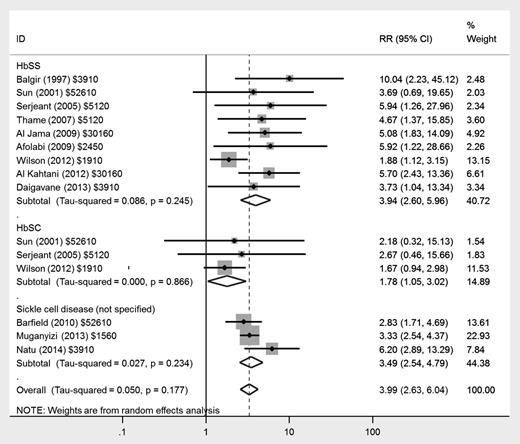
There was a fourfold increased risk of stillbirth in women with HbSS disease (RR, 3.94; 95% CI, 2.60-5.96) and an approximately twofold increased risk in women with HbSC disease (RR, 1.78; 95% CI, 1.05-3.02) compared with women without SCD (Figure 4). There was a similar increased risk of neonatal death in women with HbSS disease (RR, 2.68; 95% CI, 1.49-4.82). Only 1 study reported neonatal deaths in women with HbSC disease, giving an RR of 1.78 (95% CI, 0.52-12.41).
Forest plot of studies investigating the association of SCD with stillbirth.
The risks of preterm delivery were increased in a similar manner, with more than twofold increased risk in women with HbSS disease (RR, 2.21; 95% CI, 1.47-3.31) and a lower, nonsignificant increase in women with HbSC disease (RR, 1.45; 95% CI, 0.86-2.43) compared with women without SCD (Figure 5); there was greater heterogeneity in the studies in HbSC disease, with 1 study reporting no increase. Most studies did not differentiate between spontaneous preterm deliveries and those where labor and delivery were initiated by medical staff for maternal or fetal complications.
Forest plot of studies investigating the association of SCD with preterm delivery.
Forest plot of studies investigating the association of SCD with preterm delivery.
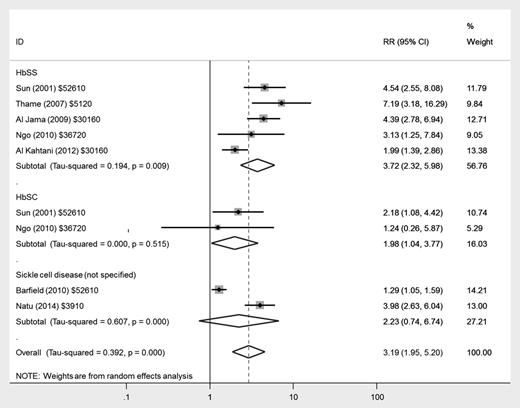
There was a nearly fourfold increased risk of infants being born small for gestational age (<10th centile) in women with HbSS disease (RR, 3.72; 95% CI, 2.32-5.98) and a twofold risk in women with HbSC disease (RR, 1.98; 95% CI, 1.04-3.77) compared with women without SCD (Figure 6); heterogeneity was low across studies.
Forest plot of studies investigating the association of SCD with delivery of small for gestational age infant.
Forest plot of studies investigating the association of SCD with delivery of small for gestational age infant.
Meta-regression demonstrated that genotype (HbSS vs HbSC) had a significant influence on adverse perinatal outcomes (stillbirth, preterm delivery, and small for gestational age infants), but not on the occurrence of preeclampsia (Table 6). There was substantially and significantly lower maternal mortality (odds ratio, 0.15; 95% CI, 0.02-0.86) and stillbirth (odds ratio, 0.28; 95% CI, 0.14-0.55) in women with SCD from countries with a gross national income of $30 000 or greater, but no significant impact of meta-regression by gross national income on preeclampsia, preterm delivery, or small for gestational age infants. There was an impact of quality of reporting, with higher odds ratios being reported for all outcomes in high-quality studies compared with low-quality studies.
Discussion
This systematic review and meta-analysis identified a strong association between SCD and adverse outcome for the mother (including maternal mortality and preeclampsia) and baby (including perinatal death, preterm delivery, and small for gestational age infants). Women with HbSS disease had significantly worse perinatal outcomes compared with those with HbSC disease, in a similar pattern to complications outside of pregnancy, and maternal mortality and stillbirth were substantially lower in high-income countries; studies rated as high quality reported increased relative risks that were generally double those of the low-quality studies. This may reflect better ascertainment of outcome data in the high-quality studies.
To the best of our knowledge, this study represents the first systematic review and meta-analysis of outcomes for pregnancies complicated by SCD. We used a comprehensive and systematic literature search, not limited by language or setting. The pooled sample size of >26 000 pregnancies in women with SCD increases the reliability of the pooled estimates for each outcome. It remains a limitation of the source data that most of the included studies were of historical design, single institution origin, and of mixed quality rating. Furthermore, a number of studies failed to define the precise genotypic diagnosis of the participants or analyze pregnancy outcomes by genotype separately. However, we tried to mitigate this limitation by stratifying the study populations into the specific genotypes (together with a SCD nonspecified group) where possible. It was also not possible to adjust the confidence intervals for the relatively small amount of clustering caused by 1.8% of women being included in the study more than once with different pregnancies, and confidence intervals might be slightly too narrow in consequence. The meta-analysis includes 5 population-wide studies; the larger sample sizes and greater number of institutions included allow increased generalizability and precision in the generation of pooled risk ratios estimates, but are subject to caution over accuracy of coding. Variation in management strategies across settings may have influenced the pregnancy outcomes; this may either have occurred through a direct impact on ameliorating pregnancy outcomes (eg, there is an uncertain benefit of prophylactic vs clinically indicated exchange transfusion in women with SCD) or through different thresholds for delivery depending on availability of neonatal care facilities (which may influence gestation of delivery in a woman with preeclampsia). Most studies included in the review did not provide comprehensive details of their management pathway for pregnant women with SCD, and the considerable heterogeneity of transfusion management plans precluded formal statistical comparison of the influence of this factor.
We limited the review and meta-analysis to well-delineated outcomes, reported across the majority of the studies as other end points (such as maternal jaundice, pulmonary hypertension, sepsis, thromboembolism, abruption, postpartum hemorrhage, and congenital fetal abnormality) were not reported consistently and the data on increased occurrence in SCD are conflicting).
With improvements in clinical management that have focused on early identification and treatment of manifestations with the aim of reducing complications, women with SCD are surviving into adulthood and actively considering reproductive options. Previous narrative reviews2,32,33 have summarized the studies, but a systematic review permits quantification of the risks for use in prepregnancy and pregnancy counseling.
It is of particular concern that women with SCD have a markedly increased risk in maternal mortality. Studies from Europe and Jamaica looking at patterns of mortality in SCD highlight acute chest syndrome, sepsis, and multiorgan failure as the 3 major causes of death.17,34 Many studies in our systematic review did not elaborate on the precise cause of maternal death or to what extent SCD contributed directly or indirectly. The risk of maternal mortality in HbSS women was quantified as being sixfold compared with women without SCD, whereas in the studies of women with HbSC disease, there was 1 maternal death (of 279 women reported), and the risk of maternal morbidity in both groups remains substantial.
There is a substantial difference in magnitude of risk of maternal death and stillbirth between countries with high and low gross national income (dichotomized at $30 000), even though the odds ratios for preeclampsia, preterm delivery, and small for gestational age infants were not significantly different, suggesting that the risk of death is dependent on the availability of adequate health care resources and expertise and the variable implementation of prophylactic or indicated exchange blood transfusion practices. This may be aggravated by the difficulties in identification of those at greatest risk of mortality; a recent Jamaican study showed that a third of those dying from multiorgan failure and acute chest syndrome had had only mild disease before the final events.34
SCD has been proposed to be a chronic inflammatory state35,36 ; endothelial damage secondary to sickled red blood cells and the subsequent release of proinflammatory cytokines may contribute to microvascular damage.37 Normal vascular, endothelial, and inflammatory adaptations in pregnancy may lead to exacerbation of these pathophysiologic changes, with manifestation of resulting maternal complications such as preeclampsia and fetal growth restriction. There are data from a small case-control study to demonstrate an association between SCD, abnormal mid-trimester uterine artery Doppler waveforms,38,39 abnormal placental histology, including placental lesions of moderate-to-severe villous sclerosis, intervillous fibrin deposits, and infarction,40 and subsequent delivery of small for gestational age infants. Prophylactic interventions to reduce preeclampsia are limited, but low-dose aspirin from mid-trimester to the end of pregnancy has been shown to decrease the occurrence of preeclampsia in other groups of high-risk women41 ; although never formally tested in a randomized controlled trial specifically in women with SCD, it is highly likely that aspirin is similarly beneficial in these women. Regular third trimester screening for fetal growth may also represent a strategy to reduce adverse outcome such as stillbirth linked to fetal growth restriction, but it is accepted that this resource would be of limited availability to less privileged health care providers.42
Further research, with appropriately powered prospective studies and strict genotype diagnosis and outcome ascertainment, is needed to understand the reasons behind the increased risks of morbidity and mortality in women with SCD, particularly those with HbSS genotype and in low income countries. There is interest in strategies such as selective prophylactic exchange transfusion43 ; the most recent Cochrane systematic review and meta-analysis of 2 trials in this population concluded that there was no clear benefit of prophylactic transfusion over a selective (emergency) approach.44 There is an urgent need for adequately powered trials of transfusion modalities in this high-risk group of pregnant women with appropriate choice of maternal and perinatal outcome measures. In high-income countries, women with SCD are usually managed by a multidisciplinary team of obstetricians, hematologists, specialist midwives, and anesthetists, but the greatest burden of morbidity and mortality undoubtedly lies within low and middle income countries, where access to specialist antenatal care may be limited. This systematic review and meta-analysis provides useful estimates of the morbidity and mortality associated with the condition and should encourage global health care policy makers, researchers, and clinicians to work together to reduce the maternal and perinatal adverse outcomes described.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors thank the librarian at the Royal College of Obstetricians and Gynaecologists (Lisa Xue) for help with obtaining some of the articles.
Authorship
Contribution: E.O.-N., L.C.C., and P.D. designed the study; E.O.-N., D.M., and L.W. conducted the literature searches; and all authors contributed to the writing of the manuscript and approved the final version.
Conflict-of-interest disclosure: J.H. has been on the advisory board for Novartis and AesRx. All other authors declare no competing financial interests.
Correspondence: Eugene Oteng-Ntim, Women’s Health Directorate, Guy’s and St Thomas’ NHS Foundation Trust, London SE1 7EH, United Kingdom; e-mail: eugene.oteng-ntim@gstt.nhs.uk.