Key Points
BMSCs from patients with TBDs are abnormal and unable to support hematopoiesis.
Knockdown of TERC with siRNA recapitulates the TBD-BMSC phenotype.
Abstract
Dyskeratosis congenita (DC) is an inherited multisystem disorder, characterized by oral leukoplakia, nail dystrophy, and abnormal skin pigmentation, as well as high rates of bone marrow (BM) failure, solid tumors, and other medical problems such as osteopenia. DC and telomere biology disorders (collectively referred to as TBD here) are caused by germline mutations in telomere biology genes leading to very short telomeres and limited proliferative potential of hematopoietic stem cells. We found that skeletal stem cells (SSCs) within the BM stromal cell population (BMSCs, also known as BM–derived mesenchymal stem cells), may contribute to the hematologic phenotype. TBD-BMSCs exhibited reduced clonogenicity, spontaneous differentiation into adipocytes and fibrotic cells, and increased senescence in vitro. Upon in vivo transplantation into mice, TBD-BMSCs failed to form bone or support hematopoiesis, unlike normal BMSCs. TERC reduction (a TBD-associated gene) in normal BMSCs by small interfering TERC-RNA (siTERC-RNA) recapitulated the TBD-BMSC phenotype by reducing proliferation and secondary colony-forming efficiency, and by accelerating senescence in vitro. Microarray profiles of control and siTERC-BMSCs showed decreased hematopoietic factors at the messenger RNA level and decreased secretion of factors at the protein level. These findings are consistent with defects in SSCs/BMSCs contributing to BM failure in TBD.
Introduction
Inherited bone marrow (BM) failure (BMF) syndromes (IBMFSs) are disorders that include the inability to make normal numbers of all or specific types of blood cells (pancytopenia, or anemia, leukopenia, and thrombocytopenia).1 Mutations in >40 genes associated with various IBMFSs have been identified to date.2 However, these genes are not specific for hematopoietic cells per se because patients with IBMFSs often have multiorgan manifestations. Dyskeratosis congenita (DC) is an IBMFS, which in its classic form is characterized not only by the triad of oral leukoplakia, nail dystrophy, and abnormal skin pigmentation, but also includes high rates of severe aplastic anemia (the main cause of death), pulmonary fibrosis, stenosis of the esophagus, urethra and/or lacrimal ducts, liver disease, premature graying of hair, and osteopenia.2 Gene mutations in subunits of telomerase (DKC1, TERT, TERC, NOP10, NHP2), and in genes involved in telomere biology (WRAP53, TINF2, CTC1, RTEL1), are associated with DC, which can be inherited in an X-linked–recessive, autosomal-dominant, or autosomal-recessive fashion.3-5 Furthermore, an appreciable minority of patients with apparent acquired aplastic anemia, but no stigmata of DC, have been found to have germline mutations in genes of the telomerase complex.6,7 Thus, patients with telomere biology disorders (which we collectively refer to as TBDs in this study) present with a broad clinical spectrum ranging from a severe multiorgan phenotype as in DC, to one more limited to BM aplasia.
BMF due to mutations in telomere biology-related genes is most likely related to improper telomere maintenance in hematopoietic stem cells (HSCs), leading to limited proliferative potential. However, the ability of HSCs to maintain a normal balance between quiescence and proliferation, self-renewal and differentiation, is based not solely on their intrinsic properties, but also on the microenvironment in which they reside, the HSC niche, as proposed by Schofield.8 As known from the work of Friedenstein and coworkers, adherent, nonhematopoietic cells of BM stroma, BM stromal cells (BMSCs, also known as BM-derived “mesenchymal stem cells”) are able to re-create the hematopoietic microenvironment (HME) upon in vivo transplantation into mice.9,10 Subsequently, a subset of multipotent skeletal stem cells (SSCs) was identified within the BMSC population, capable of re-forming a bone/marrow organ (composed of bone, hematopoiesis-supportive stroma, marrow adipocytes of donor origin; hematopoiesis of recipient origin) upon in vivo transplantation.11 Self-renewal of SSCs was also demonstrated, and their location was pinpointed to the subluminal side of marrow sinusoids (pericytes), where HSCs also reside.12
Mature osteoblastic cells,13 endothelial cells,14 and BMSCs expressing CXCL12,12,15,16 CD146,12 Nestin,17 LEPR,18 and others (reviewed in Ugarte and Forsberg19 ) have been suggested to be constituents of the hematopoietic “niche.” In the quest for an identifiable “niche”-maintaining cell, current research is directed toward perivascular SSCs/BMSCs.12,16,17,20,21 Understanding the biological properties of SSCs/BMSCs has important implications not only for skeletal physiology and disease, but also for understanding regulation of HSC physiology and dysregulation of hematopoiesis in blood disorders.
Based on the central role that SSCs/BMSCs play in skeletal physiology, we previously hypothesized that mutations affecting their function would result in a skeletal disease. Proof of principle came from examination of fibrous dysplasia of bone (FD), caused by somatic missense-activating mutations in the GNAS gene that codes for the G protein, Gsα. FD is characterized by replacement of normal lamellar bone and marrow with undermineralized woven bone and fibrotic marrow devoid of hematopoiesis.22 By using FD-BMSCs in an in vivo transplantation assay to form an ectopic ossicle, the same abnormalities seen in FD lesions were recapitulated, including a lack of hematopoiesis.23 Consistent with their role in hematopoietic support, SSCs/BMSCs can also mediate the effects of mutations on hematopoiesis, contributing to establishment of hematopoietic disease phenotypes. For example, a myeloproliferative syndrome was generated in mice in which RARγ was specifically eliminated from the HME.24 We also noted that SSCs/BMSCs from some IBMFS patients failed to re-establish the HME upon in vivo transplantation, whereas normal SSCs/BMSCs were routinely capable of doing so.25 Although BMSCs from a DC patient were reported to display morphologic abnormalities,26 direct assessment of SSC/BMSC function in TBD has not been reported. The purpose of this study was to characterize SSCs/BMSCs from patients with mutations in genes related to telomere maintenance and determine whether they contribute to BMF.
Materials and methods
BM acquisition
BM was obtained from normal donors (N, n = 13) under an institutional review board–approved protocol (National Institute of Dental and Craniofacial Research 94-D-0188, ClinicalTrials.gov NCT00001391), or from surgical waste (Office of Human Subjects Research Assurance #3113), from patients with TBD (n = 11, National Cancer Institute 02-C-0052,27 or National Heart, Lung, and Blood Institute 97-H-0041, ClinicalTrials.gov NCT00027274 and NCT00001620, respectively), and from 3 mutation-free donors related to 2 TBD patients. For comparison, BM was obtained from patients with BMF not related to telomere biology (Shwachman-Diamond syndrome [SDS, n = 4], Diamond-Blackfan anemia [DBA, n = 4], and newly diagnosed acquired aplastic anemia [AAA] patients [before treatment] without family histories or mutations in telomere-related genes [AAA, n = 8]) under institutional review board–approved protocols (02-C-0052 and 97-H-0041). All participants or their guardians provided written informed consent in accordance with Health and Human Services regulation 45 CFR 46 and the Declaration of Helsinki. Patient characteristics, their mutations, and assays performed using their cells are shown in Table 1.
Primary and secondary CFE assays
BM single-cell suspensions obtained from surgical waste or from bone fragments within the Jamshidi needle used for aspiration were used to establish cultures at clonal density (primary CFEs25 ; supplemental Methods, see supplemental Data available at the Blood Web site) to estimate the number of SSCs in BM from normal donors (n = 11), TBD patients (n = 9), SDS patients (n = 4), and DBA patients (n = 4). Secondary colony-forming efficiencies (CFEs) were determined on cells at passage (P) 1 for the same normal donors and all TBD patients, and also for AAA patients (n = 8) (supplemental Methods).
Nonclonal BMSC cultures
BM single-cell suspensions were used to establish nonclonal BMSC cultures from normal donors (N-BMSCs) and from all patients (TBD-BMSCs, SDS-BMSCs, DBA-BMSCs, and AAA-BMSCs) as described previously25 (supplemental Methods). β-Glycerophosphate (10 mM) was added to the growth medium (containing 10−8 M dexamethasone and 10−4 M ascorbic acid-2-phosphate [Dex/AscP]) for osteogenic differentiation. Cells were used between P2 and P4.
In vitro colorimetric assays
Oil red O staining was used to demonstrate adipogenesis in vitro as described previously28 (supplemental Methods) (n = 9 TBD patients). Senescence-associated (SA)-β-galactosidase staining was detected using a kit (Biovision Research Products) per the manufacturer’s protocol (supplemental Methods) (n = 6 TBD patients). A Nikon Diaphot microscope using a Plan 10/0.30 lens was used, and digital images were acquired with a Retiga 1300 camera and NIS-Elements software (QI Imaging).
qRT-PCR
Quantitative reverse transcription–polymerase chain reaction (qRT-PCR) was performed on messenger RNA (mRNA) from BMSCs as described in the supplemental Methods (n = 2 TBD patients). PPARG primers (GenBank Accession NM_138712): sense, ACGAAGACATTCCATTCACAA; antisense, CTCCACAGACACGACATTC. GAPDH primers (GenBank Accession NM_002046.3): sense, TCTCTGCTCCTCCTGTTC; antisense, GACTCCGACCTTCACCTT.
In vivo transplantation assay
BMSCs were attached to ceramic particles and transplanted subcutaneously into immunocompromised mice (2 transplants/normal or patient donors) to form an ectopic ossicle as previously described29,30 (supplemental Methods) under an institutionally approved animal study protocol. After 8 weeks, transplants were harvested and fixed for histologic evaluation with a Nikon Optiphot-2 microscope using a PlanApo 10/0.45 lens. Images were acquired with an Insight CCD camera and software (SPOT Imaging Solutions).
TERC knockdown
To mimic the defect found in TBD-BMSCs, TERC expression was reduced in N-BMSCs in 2 different ways. Initially, TERC short hairpin (sh) RNA lentiviral particles were used to generate cells with stably reduced expression of TERC. However, these cells were unable to grow. Thus, transient knockdown of TERC was performed using small interfering TERC-RNA (siTERC-RNA) (Silencer Select, predesigned and validated; Ambion) and a negative control siRNA (siNC) (Silencer negative control no. 2 siRNA; Ambion). Constructs were transfected into N-BMSCs using Lipofectamine RNAiMAX (Life Technologies), according to the manufacturer’s instructions.
Microarray analysis
RNA (5 μg), isolated from N-BMSCs, siNC-BMSCs, and siTERC-BMSCs 72 hours after transfection using an RNeasy Mini kit (Qiagen), was reverse transcribed and hybridized to an Affymetrix GeneChip Human Genome U133 Plus 2.0 array, with ∼54 000 probe sets and 1 300 000 distinct oligonucleotide features, which analyzes an expression level of ∼47 000 transcripts and variants, including 38 500 well-characterized human genes (LMT, NCI-Frederick). Three independent replicates for each experimental condition were carried out to control for intrasample variation. Genes that were under/overrepresented by more than twofold were analyzed using GeneSpring software. Signal intensity values were normalized using robust multiarray analysis summarization, and baseline transformation to median of all samples was performed. Entities were filtered based on their signal intensity values. Hierarchical clustering was performed on filtered signal intensity (>20.0), nonaveraged, fold change > 2. A fold change analysis (>10-fold) was performed to generate a list of top genes under/overrepresented between the groups.
Cytokine secretion analysis
Cytokines in conditioned medium were analyzed using a RayBio Human Cytokine Antibody Array 6 (60) according to the manufacturer’s instructions (see supplemental Methods for details).
Statistics
Statistical analyses were performed using Prism 6. Data are presented as the mean ± standard deviation. Statistical significance is indicated when P ≤ .05. For microarray analysis, the Student t test (fold change ≥ 2, corrected P ≤ .05) was used. Hierarchical clustering analyses were performed by clustering on cell type, using Pearson-centered similarity measures and a centroid linkage rule.
Results
TBD-BMSC abnormalities in vitro
CFEs (estimates of stromal stem/progenitor cells capable of density-independent clonal growth,31 ) were assessed in fresh BM single-cell suspensions from normal donors and TBD patients (n = 9 each) by counting colonies established by single colony forming units-fibroblasts. For comparison, CFEs were also determined for SDS (n = 4) and DBA (n = 4) patients (fresh aspirates from AAA patients were unavailable). CFEs for normal donors were 12.0 ± 1.2 colonies/100 000 BM-nucleated cells, comparable with previous reports.32-36 Similar results were also found for SDS and DBA, and for a single fresh AAA aspirate previously analyzed (supplemental Figure 1A). Although the range in TBD CFEs was broad, TBD cells formed only 3.6 + 5.2 colonies/100 000 cells, which is significantly lower than normal (P ≤ .0001, Figure 1A).
CFE in N-BMSCs and those derived from patients with TBD. (A) CFE of freshly isolated cells (from bone fragments from surgical waste or from Jamshidi needles) from normal donors (N, n = 11), TBD patients (n = 9, patients 1 [TERC], 2 [TINF2], 3 [RETL1], 4 [TERC], 5 [TERT], 6 [RETL1], 9 [WRAP53], 10 [no telomere-related mutation found to date], 11 [TINF2]), and from patients with other forms of BMF, SDS (n = 4), and DBA (n = 4) was determined. CFE (to date, the closest approximation of the number of skeletal stem cells within the freshly isolated BM) was reduced in TBD samples compared with normal (*P ≤ .0001), and SDS and DBA samples. A freshly isolated sample from a previous patient with AAA was also normal (see supplemental Figure 1). (B) At P1, BMSCs were assayed for secondary CFE from normal donors, and from all patients with TBD (n = 11), SDS, DBA, and AAA. TBD-BMSCs demonstrated a reduced capacity to form secondary colonies in comparison with the normal, SDS, and DBA BMSCs (*P ≤ .0001). Unlike TBD-BMSCs, BMSCs from AAA patients, who displayed a similar pattern of BMF as TBD patients, showed normal secondary CFE.
CFE in N-BMSCs and those derived from patients with TBD. (A) CFE of freshly isolated cells (from bone fragments from surgical waste or from Jamshidi needles) from normal donors (N, n = 11), TBD patients (n = 9, patients 1 [TERC], 2 [TINF2], 3 [RETL1], 4 [TERC], 5 [TERT], 6 [RETL1], 9 [WRAP53], 10 [no telomere-related mutation found to date], 11 [TINF2]), and from patients with other forms of BMF, SDS (n = 4), and DBA (n = 4) was determined. CFE (to date, the closest approximation of the number of skeletal stem cells within the freshly isolated BM) was reduced in TBD samples compared with normal (*P ≤ .0001), and SDS and DBA samples. A freshly isolated sample from a previous patient with AAA was also normal (see supplemental Figure 1). (B) At P1, BMSCs were assayed for secondary CFE from normal donors, and from all patients with TBD (n = 11), SDS, DBA, and AAA. TBD-BMSCs demonstrated a reduced capacity to form secondary colonies in comparison with the normal, SDS, and DBA BMSCs (*P ≤ .0001). Unlike TBD-BMSCs, BMSCs from AAA patients, who displayed a similar pattern of BMF as TBD patients, showed normal secondary CFE.
In preliminary experiments, we noted that TBD-BMSCs grew very poorly in medium without Dex/AscP (data not shown). Subsequent cultures were established in medium with Dex/AscP to increase proliferation and the number of cells for experiments.25 In secondary CFE assays at P1, the number of colonies increased in all samples compared with those in primary assays, due to the increased number of clonogenic, but transiently amplifying, cells generated during culture that were not present in primary CFE assays.37 However, even in the secondary assays, the CFEs of TBD-BMSCs were substantially lower than that of N-BMSCs, SDS-BMSCs, DBA-BMSCs, and AAA-BMSCs (Figure 1B).
In nonclonal cultures, TBD-BMSCs exhibited properties markedly different from those of N-BMSCs. Unlike N-BMSCs (Figure 2A), TBD-BMSCs underwent massive, spontaneous adipogenic differentiation, even when cultured under osteogenic conditions (9 of 11 TBD-BMSC cultures, Figure 2B). The adipogenic predilection of TBD-BMSCs was supported by a high level of expression of PPARG (n = 2 TBD patients) compared with N-BMSCs (Figure 2C). The remaining 2 TBD-BMSC cultures (from 2 related patients) focally deposited large amounts of collagenous matrix (Figure 2D). However, cells from 3 mutation-free siblings in this kindred also underwent fibrosis (data not shown). These clinically unaffected relatives exhibited a tendency for hypocellular marrow, and/or slightly shorter telomeres (see Table 1), presumably due to inheritance of short telomeres but not the disease-associated mutation. TBD-BMSCs from all other patients were far less fibrotic. Spontaneous adipogenic differentiation and massive fibrosis were never observed in N-BMSC cultures in basal or osteogenic differentiation medium, nor was it observed in AAA-BMSC cultures (supplemental Figure 1B).
In vitro characterization of N-BMSCs and TBD-BMSCs. (A) As N-BMSCs become confluent, they become tightly packed. (B) TBD-BMSCs spontaneously formed adipocytes as demonstrated by staining with oil red O (noted in TBD-BMSC cultures from patients 1 [TERC], 2 [TINF2], 3 [RTEL1], 4 [TERC], 5 [TERT], 6 [RTEL1], 9 [WRAP53], 10 [no mutation in known telomere-related genes found to date], 11 [TINF2]). A representative experiment is shown. (C) TBD-BMSCs express very high levels of the adipogenic master gene, PPARG (measured in triplicate from BMSCs from 2 normal donors and TBD patients 5 [TERT] and 9 [WRAP53]). A representative experiment is shown (*P ≤ .0001). (D) Unlike N-BMSCs, TBD-BMSCs from 2 related patients formed areas of very dense matrix that was contracted by the cells (noted in 2 of 11 TBD-BMSC cultures, TBD patients 7 [TERT], and 8 [TERT]). In vitro, TDB-BMSCs from the other 11 TBD patients showed far less fibrosis. (E) At P4, N-BMSCs maintained their fibroblastic morphology. (F) At ∼P4, TBD-BMSCs became large and flat, failed to achieve confluency, and stopped growing (TBD-BMSCs from all patients). (G) Little staining for SA-β-galactosidase was found in N-BMSC populations. (H) TBD-BMSC populations had substantially higher levels of SA-β-galactosidase at the same passage (TBD patients 1 [TERC], 2 [TINF2], 3 [RTEL1], 4 [TERC], 5 [TERT], 6 [RTEL1]). A representative experiment is shown. The senescent cells, with blue-green staining, were counted (1 field/chamber, 3-4 chambers/group) and the average number of positive cells is indicated (*P ≤ .05).
In vitro characterization of N-BMSCs and TBD-BMSCs. (A) As N-BMSCs become confluent, they become tightly packed. (B) TBD-BMSCs spontaneously formed adipocytes as demonstrated by staining with oil red O (noted in TBD-BMSC cultures from patients 1 [TERC], 2 [TINF2], 3 [RTEL1], 4 [TERC], 5 [TERT], 6 [RTEL1], 9 [WRAP53], 10 [no mutation in known telomere-related genes found to date], 11 [TINF2]). A representative experiment is shown. (C) TBD-BMSCs express very high levels of the adipogenic master gene, PPARG (measured in triplicate from BMSCs from 2 normal donors and TBD patients 5 [TERT] and 9 [WRAP53]). A representative experiment is shown (*P ≤ .0001). (D) Unlike N-BMSCs, TBD-BMSCs from 2 related patients formed areas of very dense matrix that was contracted by the cells (noted in 2 of 11 TBD-BMSC cultures, TBD patients 7 [TERT], and 8 [TERT]). In vitro, TDB-BMSCs from the other 11 TBD patients showed far less fibrosis. (E) At P4, N-BMSCs maintained their fibroblastic morphology. (F) At ∼P4, TBD-BMSCs became large and flat, failed to achieve confluency, and stopped growing (TBD-BMSCs from all patients). (G) Little staining for SA-β-galactosidase was found in N-BMSC populations. (H) TBD-BMSC populations had substantially higher levels of SA-β-galactosidase at the same passage (TBD patients 1 [TERC], 2 [TINF2], 3 [RTEL1], 4 [TERC], 5 [TERT], 6 [RTEL1]). A representative experiment is shown. The senescent cells, with blue-green staining, were counted (1 field/chamber, 3-4 chambers/group) and the average number of positive cells is indicated (*P ≤ .05).
N-BMSCs and AAA-BMSCs maintained their fibroblastic morphology with continued passage (Figure 2E; supplemental Figure 1B), but TBD-BMSCs increasingly displayed a senescent morphology (very large and flat). By P4, they stopped growing, and never became confluent (all TBD-BMSCs) (Figure 2F). Premature senescence of TBD-BMSCs in comparison with N-BMSCs at P4 was evident by a highly increased expression of SA-β-galactosidase (n = 2 N, 2 TBD patients) (Figure 2G-H).
TBD-BMSCs do not form bone or support hematopoiesis in vivo
When transplanted with a scaffold in vivo into immunocompromised mice (Figure 3A), N-BMSCs (even from older donors) formed copious amounts of bone, hematopoiesis-supportive stroma, and marrow adipocytes, all of donor origin (Figure 3B). Interestingly, AAA-BMSCs also formed a normal ossicle (Figure 3C), in spite of the fact that they were isolated from aplastic marrow (Figure 3D). On the other hand, TBD-BMSCs did not form bone and instead formed varying levels of fibrous tissue in association with the scaffold, and areas of varying sizes were filled with adipocytes (Figure 3E-F). Hematopoiesis did not colonize the transplants (n = 8 TBD patients in duplicate). These in vivo results confirmed the results found in vitro: massive adipogenesis and fibrosis (in some instances) by TBD-BMSCs. Furthermore, TBD-BMSC–generated transplants reflect not only a skeletal aspect of TBD (osteopenia, avascular necrosis of the hip, fracture), but also the marrow aplasia as in native TBD marrow (Figure 3G).
In vivo transplantation of N-BMSCs and TBD-BMSCs. (A) Cells (N-BMSCs, AAA-BMSCs, and TBD-BMSCs) were attached to hydroxyapatite/tricalcium phosphate ceramic particles as a scaffold (labeled “s” in panels B-G) and transplanted under the skin of immunocompromised mice. After 8 weeks, the transplants were harvested, fixed and decalcified, and used for histologic examination after H&E staining. (B) When N-BMSCs (even from donors of advanced age) were transplanted, they formed an ectopic bone/marrow organ, with bone (labeled “b”), hematopoiesis-supportive stroma (which cannot be seen due to the high density of hematopoietic cells), and marrow adipocytes (labeled “a”) of human origin, whereas hematopoiesis (labeled “hp”) was of mouse origin (n = 8 different normal donors, 2 transplants/donor). (C) Similar bone/marrow organs were formed by AAA-BMSCs (n = 8 different AAA donors, 2 transplants/donor), although these cells were derived from aplastic marrow (D) (AAA Pt - NHLBI-64-1). (E-F) Under the same conditions, TBD-BMSCs were not capable of forming bone or of supporting hematopoiesis, but instead formed dense fibrotic tissue (labeled “ft”) in association with the scaffold. In addition, extensive areas of marrow adipocytes (labeled “a”) were noted in the TBD-BMSC transplants (n = 8 donors, 2 transplants each for TBD patients 1 [TERC], 2 [TINF2], 3 [RTEL1], 4 [TERC], 5 [TERT], 6 [RTEL1], 9 [WRAP53], 10 [no mutation in known telomere-related genes found to date], 11 [TINF2]). (G) The transplants generated by TBD-BMSCs are highly reminiscent of a BM biopsy taken from TBD patient 5 (TERT), showing marrow aplasia and large fields of adipocytes. (Biopsy images were obtained using an Olympus BX-41 microscope with UPlan 10×/0.30 or Uplan 20×/0.50 lenses, and an Olympus DP72 digital camera and software [Olympus], courtesy of Dr. Irina Maric, Warren G. Magnuson Clinical Center, NIH, DHHS).
In vivo transplantation of N-BMSCs and TBD-BMSCs. (A) Cells (N-BMSCs, AAA-BMSCs, and TBD-BMSCs) were attached to hydroxyapatite/tricalcium phosphate ceramic particles as a scaffold (labeled “s” in panels B-G) and transplanted under the skin of immunocompromised mice. After 8 weeks, the transplants were harvested, fixed and decalcified, and used for histologic examination after H&E staining. (B) When N-BMSCs (even from donors of advanced age) were transplanted, they formed an ectopic bone/marrow organ, with bone (labeled “b”), hematopoiesis-supportive stroma (which cannot be seen due to the high density of hematopoietic cells), and marrow adipocytes (labeled “a”) of human origin, whereas hematopoiesis (labeled “hp”) was of mouse origin (n = 8 different normal donors, 2 transplants/donor). (C) Similar bone/marrow organs were formed by AAA-BMSCs (n = 8 different AAA donors, 2 transplants/donor), although these cells were derived from aplastic marrow (D) (AAA Pt - NHLBI-64-1). (E-F) Under the same conditions, TBD-BMSCs were not capable of forming bone or of supporting hematopoiesis, but instead formed dense fibrotic tissue (labeled “ft”) in association with the scaffold. In addition, extensive areas of marrow adipocytes (labeled “a”) were noted in the TBD-BMSC transplants (n = 8 donors, 2 transplants each for TBD patients 1 [TERC], 2 [TINF2], 3 [RTEL1], 4 [TERC], 5 [TERT], 6 [RTEL1], 9 [WRAP53], 10 [no mutation in known telomere-related genes found to date], 11 [TINF2]). (G) The transplants generated by TBD-BMSCs are highly reminiscent of a BM biopsy taken from TBD patient 5 (TERT), showing marrow aplasia and large fields of adipocytes. (Biopsy images were obtained using an Olympus BX-41 microscope with UPlan 10×/0.30 or Uplan 20×/0.50 lenses, and an Olympus DP72 digital camera and software [Olympus], courtesy of Dr. Irina Maric, Warren G. Magnuson Clinical Center, NIH, DHHS).
TERC knockdown in N-BMSCs mimics the TBD-BMSC phenotype in vitro
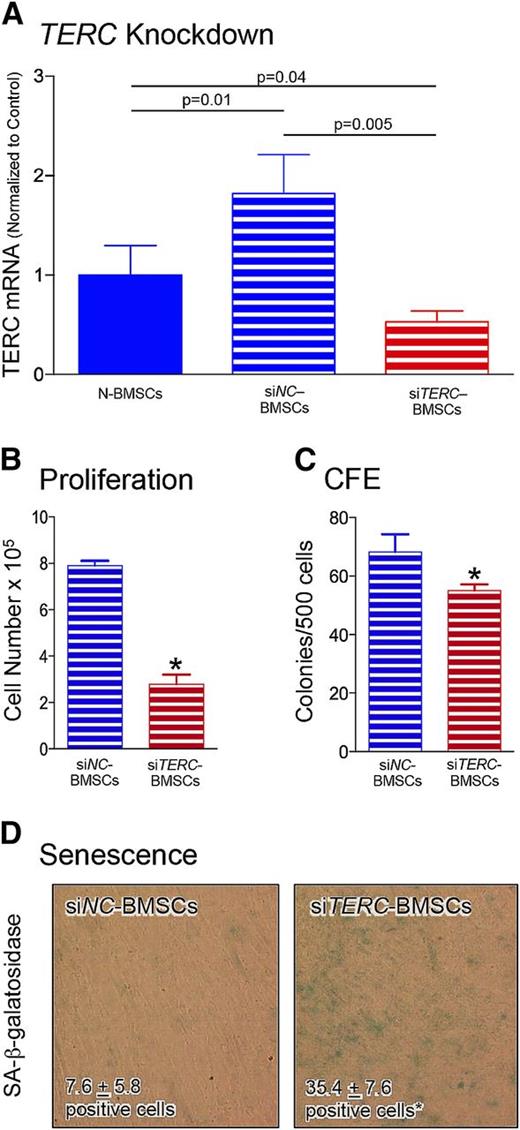
Knockdown of TERC (a gene mutated in some of our patients) was used to determine whether a reduction in TERC was able to establish a TBD-like phenotype in N-BMSCs. Attempts to create BMSC lines stably deficient in TERC through the use of shRNA were unsuccessful due to the lack of cell proliferation (data not shown). Consequently, an siRNA approach was pursued. Although there was a significant increase in TERC mRNA levels in transfection control (siNC) BMSCs compared with N-BMSCs, TERC was significantly reduced by siTERC-RNA compared with both N-BMSCs and siNC-BMSCs (Figure 4A).
Reduction of TERC by siRNA. (A) To determine whether reduction in TERC could mimic the characteristics observed in TBD-BMSCs, transient reduction of TERC utilizing siRNA was used using a commercially available negative control (siNC-RNA) and siTERC-RNA (siTERC) constructs. Although there was a significant increase in TERC mRNA levels in transfection control (siNC-treated) BMSCs compared with N-BMSCs, TERC was significantly reduced by siTERC-RNA compared with both N-BMSCs and siNC-BMSCs after 72 hrs (n = 6 different N-BMSC cultures, and n = 3 different cultures each for siNC and siTERT treatment). (B) Seventy-two hours after transfection, the cells were trypsinized and plated at 50 000 cells/well of a 6-well plate (nonclonal density), allowed to grow for 7 days, and the number of cells was counted. TERC reduction substantially lowered the proliferation of siTERC-BMSCs compared with siNC-BMSCs (n = 3 different siNC-BMSC and siTERC-BMSC cultures). (C) Likewise, when transfected cells were trypsinized and plated at 500 cells/100 cm2 dish (clonal density), the secondary CFE determined after 14 days was also reduced in the siTERC-BMSCs compared with siNC-BMSCs, in spite of the transient nature of the TERC reduction. (D) Seventy-two hours after transfection, siTERC-BMSCs also displayed significantly more SA-β-galactosidase compared with siNC-BMSCs (n = 2 experiments performed in triplicate from 2 different siNC-BMSC and siTERC-BMSC cultures). A representative experiment is shown. The senescent cells, with blue-green staining, were counted (1 field/chamber, 3-4 chambers/group) and the average cell number of positive cells is indicated (*P ≤ .05).
Reduction of TERC by siRNA. (A) To determine whether reduction in TERC could mimic the characteristics observed in TBD-BMSCs, transient reduction of TERC utilizing siRNA was used using a commercially available negative control (siNC-RNA) and siTERC-RNA (siTERC) constructs. Although there was a significant increase in TERC mRNA levels in transfection control (siNC-treated) BMSCs compared with N-BMSCs, TERC was significantly reduced by siTERC-RNA compared with both N-BMSCs and siNC-BMSCs after 72 hrs (n = 6 different N-BMSC cultures, and n = 3 different cultures each for siNC and siTERT treatment). (B) Seventy-two hours after transfection, the cells were trypsinized and plated at 50 000 cells/well of a 6-well plate (nonclonal density), allowed to grow for 7 days, and the number of cells was counted. TERC reduction substantially lowered the proliferation of siTERC-BMSCs compared with siNC-BMSCs (n = 3 different siNC-BMSC and siTERC-BMSC cultures). (C) Likewise, when transfected cells were trypsinized and plated at 500 cells/100 cm2 dish (clonal density), the secondary CFE determined after 14 days was also reduced in the siTERC-BMSCs compared with siNC-BMSCs, in spite of the transient nature of the TERC reduction. (D) Seventy-two hours after transfection, siTERC-BMSCs also displayed significantly more SA-β-galactosidase compared with siNC-BMSCs (n = 2 experiments performed in triplicate from 2 different siNC-BMSC and siTERC-BMSC cultures). A representative experiment is shown. The senescent cells, with blue-green staining, were counted (1 field/chamber, 3-4 chambers/group) and the average cell number of positive cells is indicated (*P ≤ .05).
When siTERC-BMSCs were replated 72 hours after treatment and allowed to proliferate for 7 days, a dramatic decrease was noted (Figure 4B). When the cells were replated for a secondary CFE assay, the CFE of siTERC-BMSCs was also significantly reduced (Figure 4C), whereas the CFE of siNC-BMSCs remained normal (compare with N-BMSCs in Figure 1B). After replating, a large increase in the number of senescent cells in siTERC-BMSCs was noted in comparison with siNC-BMSCs (Figure 4D). Although it would be desirable to use siTERC-BMSCs for the in vivo transplantation assay, this was not feasible due to the transient nature of siRNA inhibition (ectopic ossicles take 4 to 8 weeks to become established). Nonetheless, these results indicate that even transient TERC deficiency induces N-BMSCs to behave similar to TBD-BMSCs in vitro.
Transient TERC knockdown rapidly alters gene expression in N-BMSCs
Sufficient numbers of TBD-BMSCs could not be obtained for microarray analysis due to poor proliferation and early senescence. Therefore, siTERC-BMSCs were used as a model based on their similarity to TBD-BMSCs as shown in Figure 4. Microarray analysis was performed on RNA extracted after 72 hours of treatment from N-BMSCs, siNC-BMSCs, and siTERC-BMSCs. The global gene expression showed a distinct pattern of under/overrepresented genes in the siTERC-BMSCs, compared with siNC-BMSCs and N-BMSCs, which clustered together (Figure 5A). Analysis of genes that were altered more than fourfold revealed a decrease in expression of CXCL12 (SDF-1, stromal-derived factor 1) (Figure 5B), a BMSC-derived chemokine related to migration of HSCs,38 and regarded as a marker of “niche”-maintaining stromal cells. In further analysis of genes that were altered by twofold (supplemental Table 1), angiopoietin 1, known to play an important role in maintenance of the HSC niche,12,39 was significantly underrepresented, as were other factors such as hepatocyte growth factor and interleukin-6 (IL-6). KITL (KIT ligand, stem cell factor [SCF]) was also significantly altered, but by less than twofold (Figure 5C). Consistent with the osteogenic defect noted in TBD-BMSCs upon in vivo transplantation, the Wnt inhibitor, DKK1, was significantly overrepresented (Figure 5C), whereas WISP1, a positive regulator of Wnt signaling, was significantly underrepresented (supplemental Table 1). Other members of the Wnt signaling pathway were altered (supplemental Figure 2), as were lipoprotein biosynthetic pathways and membrane lipid biosynthetic processes (supplemental Figures 3-4). However, after 72 hours of TERC knockdown, PPARG was not significantly altered (data not shown), unlike upregulation noted in TBD-BMSCs.
Microarray analysis of BMSCs with reduced TERC expression. (A) Global gene expression patterns exhibited by negative control siNC-BMSCs (NC), untreated N-BMSCs (N), and siTERC-BMSCs (T). In unsupervised hierarchical clustering, NC and N cluster together. Although there are differences between the NC and N patterns, there are clearly genes that are underrepresented (−) and overrepresented (+) in the T pattern compared with NC and N (n = 3 different N, NC and T cultures). (B) Comparison of the top under- and overrepresented genes with greater than a fourfold difference in siTERC-BMSCs reveals that CXCL12 (SDF-1) is reduced in these cells compared with siNC-BMSCs and N-BMSCs cells. (C) Further analysis of the genes showing a twofold change in siTERC-BMSCs (see supplemental Table 1), indicates a reduction in genes associated with hematopoiesis (again, CXCL12, and also Angiopoietin 1, Hepatocyte Growth Factor, and IL6. KITL (SCF) was also statistically lower in T cells than N and NC cells, although there was less than a twofold difference. The negative regulator of the Wnt, DKK1, was statistically higher in T cells than in N and NC cells (*P ≤ .05).
Microarray analysis of BMSCs with reduced TERC expression. (A) Global gene expression patterns exhibited by negative control siNC-BMSCs (NC), untreated N-BMSCs (N), and siTERC-BMSCs (T). In unsupervised hierarchical clustering, NC and N cluster together. Although there are differences between the NC and N patterns, there are clearly genes that are underrepresented (−) and overrepresented (+) in the T pattern compared with NC and N (n = 3 different N, NC and T cultures). (B) Comparison of the top under- and overrepresented genes with greater than a fourfold difference in siTERC-BMSCs reveals that CXCL12 (SDF-1) is reduced in these cells compared with siNC-BMSCs and N-BMSCs cells. (C) Further analysis of the genes showing a twofold change in siTERC-BMSCs (see supplemental Table 1), indicates a reduction in genes associated with hematopoiesis (again, CXCL12, and also Angiopoietin 1, Hepatocyte Growth Factor, and IL6. KITL (SCF) was also statistically lower in T cells than N and NC cells, although there was less than a twofold difference. The negative regulator of the Wnt, DKK1, was statistically higher in T cells than in N and NC cells (*P ≤ .05).
Transient TERC knockdown decreased secretion of hematopoiesis-supportive factors
Serum-free conditioned media generated by N-BMSCs, siNC-BMSCs, and siTERC-BMSCs were analyzed for cytokine content using a commercial cytokine array (supplemental Figure 5). TERC knockdown resulted in decreases (between 18% and 38%) in SCF (KITL), SDF-1 (the ligand for CXCL12), angiogenin (ribonuclease 5), and IL-6 in comparison with NC-BMSCs, and also angiogenin and IL-6 in comparison with N-BMSCs (Figure 6B). These results indicate that changes in gene expression that impact on hematopoietic support induced by TERC knockdown can also be detected at the protein level.
Cytokine analysis of conditioned medium generated by BMSCs with reduced TERC expression. (A) A commercial cytokine array (RayBio Human Cytokine Antibody Array 6 [60]) was used to assess the levels of cytokines secreted into the medium at 72 hours by untreated N-BMSCs, and 72 hours after transfection with negative control-siRNA, (siNC-BMSCs) and siTERC-RNA (siTERC-BMSCs) (n = 4 for each of the 3 groups). (B) A number of cytokines associated with hematopoiesis such as SCF, SDF-1, Angiogenin, and IL6 were found to be decreased between 18% and 30% in siTERC-BMSCs compared with siNC-BMSCs, although changes in SCF and SDF-1 were not significant when compared with N-BMSCs (*P ≤ .05).
Cytokine analysis of conditioned medium generated by BMSCs with reduced TERC expression. (A) A commercial cytokine array (RayBio Human Cytokine Antibody Array 6 [60]) was used to assess the levels of cytokines secreted into the medium at 72 hours by untreated N-BMSCs, and 72 hours after transfection with negative control-siRNA, (siNC-BMSCs) and siTERC-RNA (siTERC-BMSCs) (n = 4 for each of the 3 groups). (B) A number of cytokines associated with hematopoiesis such as SCF, SDF-1, Angiogenin, and IL6 were found to be decreased between 18% and 30% in siTERC-BMSCs compared with siNC-BMSCs, although changes in SCF and SDF-1 were not significant when compared with N-BMSCs (*P ≤ .05).
Discussion
In this study, we demonstrated that TBD-BMSCs display profound differences compared with N-BMSCs. In vitro, TBD-BMSCs had decreased CFE, indicating a reduced frequency of clonogenic SSCs/BMSCs, as has been found in TERC-deficient mice.40,41 Decreased CFE was also reflected by poor proliferation of TBD-BMSCs, along with accelerated senescence, which has also been described in TERC-deficient mice.40,41 Furthermore, TBD-BMSCs exhibited changes in differentiation potential, reflected by their spontaneous differentiation into adipocytes, and in some cases, collagen-depositing fibroblasts (2 patients from 1 kindred), both in vitro and in vivo. However, fibrosis was noted in cells from mutation-free siblings in that kindred, therefore we cannot rule out a non-TBD cause, but we report the findings here based on the fact that many patients with TBD exhibit pulmonary and liver fibrosis. Transplantation of TBD-BMSCs resulted in development of “nonossicles,” composed of fibrotic tissue, no bone, extensive areas of adipocytes, and devoid of hematopoiesis. The overall histology of these transplants mirrored directly, on the one hand, the spontaneous adipogenesis and fibrosis seen in TBD-BMSC cultures, and on the other, acellular fatty marrow as seen in bone biopsies from TBD patients. Interestingly, AAA-BMSCs from patients with nonfamilial AAA were indistinguishable from N-BMSCs in their ability to grow and establish a complete bone/marrow organ. They did not reproduce the native aplastic marrow histology, reflecting the bystander role of stroma in which an immune attack of hematopoietic cells and progenitors leaves the function of stromal cells unscathed.42 Although it is possible that the abnormalities seen in TBD-BMSCs resulted from lack of normal signals from hematopoietic cells in the abnormal marrow, these results strongly suggest that the defect noted in TBD-BMSCs is intrinsic to the cells themselves.
Reduced proliferation observed in TBD-BMSCs was directly mimicked by TERC knockdown in N-BMSCs, suggesting that the inherent phenotype of TBD-BMSCs with telomerase mutations was not only consistent with, but actually dependent on, their reduced telomerase activity. TERC knockdown in N-BMSCs induced profound changes in the expression of genes implicated in HME/niche function, such as CXCL12 and ANGIOPOIETIN-1. Likewise, DKK1 overexpression, implicated in induction of adipogenesis in BMSCs, was observed in siTERC-BMSCs, and PPARG overexpression in TBD-BMSCs, suggesting that induction of spontaneous adipogenesis can in turn be linked to reduced telomerase activity.
Altogether, these data establish a link between telomere maintenance in SSCs/BMSCs and their ability to differentiate into diverse stromal “lineages” such as osteoblasts, adipocytes, or fibroblasts. Reduced telomere maintenance leads to reduced frequency of clonogenic progenitors (most likely due to their inability to self-renew as recently demonstrated in induced pluripotent stem cells derived from DC patients43 ), and reduced proliferation and premature senescence of nonclonal populations. It also appears to abate their osteogenic capacity and to promote their adipogenic and fibrogenic capacity as assayed by in vivo transplantation. The inherent ability of SSCs/BMSCs to generate distinct lineages that belong to the natural bone/marrow organ is thus, in a way, synchronized with their growth history, inasmuch as senescence selects differentiation into certain lineages over others. The appearance of distinct stromal lineages (osteogenic and adipogenic) at distinct developmental times in individual skeletal segments is well known,44 and it was suggested previously that the distinct stromal lineages (osteogenic and adipogenic) are better conceptualized as distinct differentiation capacities acquired by a common stromal progenitor along a temporal sequence, rather than as an array of differentiation pathways that can be simultaneously elicited upon exposure to distinct signals/cues.45
Importantly, our data link the replicative properties of SSCs/BMSCs with their ability to establish the HME in vivo. Recreation of the HME by SSCs/BMSCs in vivo was shown to be dependent upon establishment of sinusoids and self-renewal of transplanted cells into sinusoid-associated CD146-expressing stromal cells.12 This provides a plausible connection between decreased proliferation and inability to transfer the HME, which our data support. TBD-BMSCs with impaired telomere maintenance and decreased proliferation fail to transfer the HME, bolstering the view that the ability of SSCs to self-renew is linked to their ability to host and retain hematopoiesis and HSCs.
Massive spontaneous adipogenesis in vitro and generation of excess adipose tissue at the expense of bone in ectopic ossicles represent complementary features of the abnormal function of TBD-BMSCs, mirror one another in 2 different experimental settings, and together mirror what one observes in TBD BM in situ. These data suggest a link between cellular senescence and adipogenesis in SSCs/BMSCs. The broad developmental scenario that provides the context for physiological marrow adipogenesis in vivo is consistent with the notion that adipogenesis and cell senescence are related to one another. Marrow adipogenesis is an entirely postnatal event, progresses during skeletal growth, and increases as a function of age after skeletal maturity. Under specific conditions, adipocytes may behave as negative regulators of hematopoiesis,46 leading to an inverse relationship between adipocyte content and hematopoietic cell mass at specific skeletal sites. Even in acquired aplastic anemia without telomere-related defects, adipocytes fill the void produced following hematopoietic destruction.47 We have shown that senescence induced by TERC knockdown involves changes in expression of genes pivotal to hematopoietic regulation by BMSCs, such as CXCL12 and ANGIOPOIETIN-1, consistent with the idea that senescence of SSCs/BMSCs changes the composition and function of bone as an organ.
Short-term TERC knockdown by siRNA treatment of N-BMSCs caused changes that are consistent with the TBD-BMSC phenotype displayed in vitro and in vivo. However, based on a population doubling time between 40 and 80 hours for N-BMSCs,48-50 the changes noted in siTERC-BMSCs cannot be attributed to poor telomere maintenance, but must stem from a decrease in telomerase activity due to reduced TERC expression. Evidence is emerging for a role of telomerase activity in stem cell biology that does not involve its role in telomere maintenance51 ; for example, telomerase appears to modulate the Wnt–β-catenin signaling pathway in epidermal stem cells.52 To date, virtually all studies on nontelomeric functions of telomerase have focused on TERT, which has been identified as a transcriptional regulator of a number of genes.51 However, TERT expression was unaltered by TERC knockdown in our study, and in TERC-null mice.53 TERC may also have nontelomeric effects, based on changes observed after only 72 hours of TERC knockdown, and as suggested by profound defects in hematopoietic differentiation of induced pluripotent stem cells derived from patients with TERC mutations, independent of telomere length.54
The strengths of our study lie in the ability to interrogate the function of TBD-SSCs/BMSCs in vitro, but more importantly, by in vivo transplantation, whereby the ability of the cells to support hematopoiesis was directly assessed. Furthermore, we were able to recapitulate a number of TBD-like changes in N-BMSCs by TERC knockdown, allowing us to begin to assess how TERC deficiency modulates SSC/BMSC function, particularly by analysis of factors that support hematopoiesis, adipogenesis, and osteogenesis. The limitations of our study are based on the fact that our TBD patients are heterogeneous in age and mutations, and the limited number of patients with the same genotype precluded a stringent genotype/phenotype correlation. In addition, due to their low frequency in BM, BMSCs were ex vivo–expanded to perform the study, which may lead to in vitro proliferative stress in TBD-BMSCs. However, such stress may also be clinically relevant in consideration of growth and maintenance of marrow in vivo.
In conclusion, our study shows that SSCs/BMSCs, responsible for the creation of the HME, may contribute (along with the defect in HSCs) to the TBD phenotype and BMF. This may also account for the lack of early engraftment, or BMF years later, noted in some TBD patients following allogeneic BM transplantation.55 The notion that BMSCs may be involved in certain genetic hematologic disorders is not new, as exemplified by the classically known hematologic defects in steel factor (KITL, SCF)-deficient mice.56 However, the defect in those mice could not be pinpointed to SSCs/BMSCs directly, based on steel factor expression by other cell types,57 an issue averted by our transplantation assay. Our study highlights the role of the specific experimental layout provided by heterotopic transplantation of SSCs/BMSCs for dissecting intrinsic dysfunction of their hematopoiesis-supportive function. Our data also strengthen the emerging view that SSCs/BMSCs may represent targets of therapeutic intervention in specific hematologic disorders emanating from an altered interplay between hematopoietic and stromal progenitors.
The microarray data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE64023).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Irina Maric (Warren G. Magnuson Clinical Center, National Institutes of Health [NIH], US Department of Health and Human Services [DHHS]) for providing the images of DC and AAA BM biopsies. The authors also thank all study participants for their valuable contributions.
This work was supported by the Intramural Research Program of the NIH, DHHS (the National Institute of Dental and Craniofacial Research Division of Intramural Research: A.B., S.Y., N.C., S.A.K., P.G.R.; the National Cancer Institute Cancer Research Center: P.J.M., G.M.; the National Cancer Institute Division of Cancer Epidemiology and Genetics: N.G., S.A.S., B.P.A.; the National Heart, Lung, and Blood Institute Intramural Research Program: B.D., C.E.D., N.S.Y.); and by GGP09227 Telethon, European Union Pluripotent Stem Cell Resources for Mesodermal Medicine - 60243, Ministry of Education, Universities and Research (PRIN 2010-2011, 20102M7T8X; P.B.).
Authorship
Contribution: A.B., P.J.M., E.P., S.Y., B.J.S., N.C., S.A.K., G.M., and B.D. performed experiments, collected, analyzed, and interpreted data, and assembled data for presentation; N.G., S.A.S., C.E.D., N.S.Y., and B.P.A. obtained patient samples and interpreted data; P.B. analyzed and interpreted data; P.G.R. analyzed and interpreted data and assembled data for presentation and for publication.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for A.B. is Merck Research Laboratories, RY34-B270, 126 East Lincoln Ave, Rahway, NJ 07065. The current affiliation for E.P. is Amgen, One Amgen Center Dr, Thousand Oaks, CA 91320. The current affiliation for S.Y. is University of Pittsburgh, 571 Salk Hall, 3501 Terrace St, Pittsburgh, PA 15261. The current affiliation for B.J.S. is Mount Sinai School of Medicine, 1 Gustave L. Levy Pl, New York, NY 10029.
Correspondence: Pamela G. Robey, Craniofacial and Skeletal Diseases Branch, National Institute of Dental and Craniofacial Research, National Institutes of Health, Department of Health and Human Services, 30 Convent Dr MSC 4320, Bethesda, MD 20892; e-mail: probey@dir.nidcr.nih.gov.

![Figure 1. CFE in N-BMSCs and those derived from patients with TBD. (A) CFE of freshly isolated cells (from bone fragments from surgical waste or from Jamshidi needles) from normal donors (N, n = 11), TBD patients (n = 9, patients 1 [TERC], 2 [TINF2], 3 [RETL1], 4 [TERC], 5 [TERT], 6 [RETL1], 9 [WRAP53], 10 [no telomere-related mutation found to date], 11 [TINF2]), and from patients with other forms of BMF, SDS (n = 4), and DBA (n = 4) was determined. CFE (to date, the closest approximation of the number of skeletal stem cells within the freshly isolated BM) was reduced in TBD samples compared with normal (*P ≤ .0001), and SDS and DBA samples. A freshly isolated sample from a previous patient with AAA was also normal (see supplemental Figure 1). (B) At P1, BMSCs were assayed for secondary CFE from normal donors, and from all patients with TBD (n = 11), SDS, DBA, and AAA. TBD-BMSCs demonstrated a reduced capacity to form secondary colonies in comparison with the normal, SDS, and DBA BMSCs (*P ≤ .0001). Unlike TBD-BMSCs, BMSCs from AAA patients, who displayed a similar pattern of BMF as TBD patients, showed normal secondary CFE.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/5/10.1182_blood-2014-06-566810/4/m_793f1.jpeg?Expires=1765088987&Signature=pbVJzXWozQ9QfqIPGKi3rHc3gsPxEA~q0szNVeksI7CDnr-nvdtNNP2n7NrqdTIZ4RX7O4qQKDVNocuJGhFMprrdzHltlx0JYw3bkZxKpSBCO9C4EO0klAm~DIePB1lOV3baIDr3T~pQ8u2nUiE8Mym6V1R8fLNAaTdY4lsTOQnEyNqRBh6yw2DBLDM9RZnJcknzs5kloPs75utHD2-OhyamOu1~h34Os2F4fI1Vdky-Pd2B312QMlB-heMVrvJYNzHPUzU0eAXFm9LCaBJMpC9VBjVNoY5RTaTW0vtNP8iA1Oryqw1PDXrnOX3DgBQUo6PkhXhorycSMiWR70voIw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. In vitro characterization of N-BMSCs and TBD-BMSCs. (A) As N-BMSCs become confluent, they become tightly packed. (B) TBD-BMSCs spontaneously formed adipocytes as demonstrated by staining with oil red O (noted in TBD-BMSC cultures from patients 1 [TERC], 2 [TINF2], 3 [RTEL1], 4 [TERC], 5 [TERT], 6 [RTEL1], 9 [WRAP53], 10 [no mutation in known telomere-related genes found to date], 11 [TINF2]). A representative experiment is shown. (C) TBD-BMSCs express very high levels of the adipogenic master gene, PPARG (measured in triplicate from BMSCs from 2 normal donors and TBD patients 5 [TERT] and 9 [WRAP53]). A representative experiment is shown (*P ≤ .0001). (D) Unlike N-BMSCs, TBD-BMSCs from 2 related patients formed areas of very dense matrix that was contracted by the cells (noted in 2 of 11 TBD-BMSC cultures, TBD patients 7 [TERT], and 8 [TERT]). In vitro, TDB-BMSCs from the other 11 TBD patients showed far less fibrosis. (E) At P4, N-BMSCs maintained their fibroblastic morphology. (F) At ∼P4, TBD-BMSCs became large and flat, failed to achieve confluency, and stopped growing (TBD-BMSCs from all patients). (G) Little staining for SA-β-galactosidase was found in N-BMSC populations. (H) TBD-BMSC populations had substantially higher levels of SA-β-galactosidase at the same passage (TBD patients 1 [TERC], 2 [TINF2], 3 [RTEL1], 4 [TERC], 5 [TERT], 6 [RTEL1]). A representative experiment is shown. The senescent cells, with blue-green staining, were counted (1 field/chamber, 3-4 chambers/group) and the average number of positive cells is indicated (*P ≤ .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/5/10.1182_blood-2014-06-566810/4/m_793f2.jpeg?Expires=1765088987&Signature=gjFZKAmai9i-jMPAqJKtU2oLHB~RwfBoYRD95eDLCNcHyYArdGIncsvhqF7PaYh8HlRiyyIuNCBDa95TltLM-dpORFMxQwmErpl4iOMJKS1hsf~3mbiBCTNj9ZI7yTRKHR-AZmq57uHvlxXS~1XYQrAk97uYpapekCJD55FpSd~NARy9GJHEY5sTrcPz8PjEbIndrzpLs4WDXaf~ZaPDJikarwNM9XM5lE2RdZhA3tsEt-qL94AkEVpvhFzOpXLhV81xmmoi17lgdyO1VV1n3QiJhuO2KRhhNIaerQB0ToYec81a4K1DpAKr9HF7t7HsyLRdge~ifGZArKnW~w8CLA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. In vivo transplantation of N-BMSCs and TBD-BMSCs. (A) Cells (N-BMSCs, AAA-BMSCs, and TBD-BMSCs) were attached to hydroxyapatite/tricalcium phosphate ceramic particles as a scaffold (labeled “s” in panels B-G) and transplanted under the skin of immunocompromised mice. After 8 weeks, the transplants were harvested, fixed and decalcified, and used for histologic examination after H&E staining. (B) When N-BMSCs (even from donors of advanced age) were transplanted, they formed an ectopic bone/marrow organ, with bone (labeled “b”), hematopoiesis-supportive stroma (which cannot be seen due to the high density of hematopoietic cells), and marrow adipocytes (labeled “a”) of human origin, whereas hematopoiesis (labeled “hp”) was of mouse origin (n = 8 different normal donors, 2 transplants/donor). (C) Similar bone/marrow organs were formed by AAA-BMSCs (n = 8 different AAA donors, 2 transplants/donor), although these cells were derived from aplastic marrow (D) (AAA Pt - NHLBI-64-1). (E-F) Under the same conditions, TBD-BMSCs were not capable of forming bone or of supporting hematopoiesis, but instead formed dense fibrotic tissue (labeled “ft”) in association with the scaffold. In addition, extensive areas of marrow adipocytes (labeled “a”) were noted in the TBD-BMSC transplants (n = 8 donors, 2 transplants each for TBD patients 1 [TERC], 2 [TINF2], 3 [RTEL1], 4 [TERC], 5 [TERT], 6 [RTEL1], 9 [WRAP53], 10 [no mutation in known telomere-related genes found to date], 11 [TINF2]). (G) The transplants generated by TBD-BMSCs are highly reminiscent of a BM biopsy taken from TBD patient 5 (TERT), showing marrow aplasia and large fields of adipocytes. (Biopsy images were obtained using an Olympus BX-41 microscope with UPlan 10×/0.30 or Uplan 20×/0.50 lenses, and an Olympus DP72 digital camera and software [Olympus], courtesy of Dr. Irina Maric, Warren G. Magnuson Clinical Center, NIH, DHHS).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/5/10.1182_blood-2014-06-566810/4/m_793f3.jpeg?Expires=1765088987&Signature=sRw45eODy46pK2KFobkxMe~DUjh5b4ocKg7hR6mEM~s4umDIp8hLYnc5Knw9ssEffXqH7SofgxtHuR22D3OHaoX2x9PftBKudO5fWxiP9lOf~G~t~0t5AIXZUI7lQPxjMZMxOxggnugjcEXN5K2HKQOngpV3wFZYm2tmrfh6uvS2Yv1Djlk8HrMdmu5YAGul7UADo7PlDcC6wcfVaFngmBTreV3VVWIbqDaRd8DqSpkQQKwIkuJnFpP9DAT-ylzn9YEc1qw8ocaG916WLePWEqUhoggqG7uoea5gns-svhfDlz-YsKt0imXHknq6CGwb5e7Kqd2pywnGZkIHoUkOnA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. Cytokine analysis of conditioned medium generated by BMSCs with reduced TERC expression. (A) A commercial cytokine array (RayBio Human Cytokine Antibody Array 6 [60]) was used to assess the levels of cytokines secreted into the medium at 72 hours by untreated N-BMSCs, and 72 hours after transfection with negative control-siRNA, (siNC-BMSCs) and siTERC-RNA (siTERC-BMSCs) (n = 4 for each of the 3 groups). (B) A number of cytokines associated with hematopoiesis such as SCF, SDF-1, Angiogenin, and IL6 were found to be decreased between 18% and 30% in siTERC-BMSCs compared with siNC-BMSCs, although changes in SCF and SDF-1 were not significant when compared with N-BMSCs (*P ≤ .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/5/10.1182_blood-2014-06-566810/4/m_793f6.jpeg?Expires=1765088987&Signature=q3sGNtalruhDRqjEyJkqdvEMlUnK3uS2ESv1OOjuLh5x37PqO-cys7WVJ26m4j3OaCwChJbGIsaUKC4EsCru2wE23cahrUkOrQ4WwBooZoyQ-BxcQgga55DFOjB-IrBcT3v7xWa4QIWySoBC9itSGUEJQJXLjdS1vAJJTdgLuqxaQIF0ejdnsPIOt1nI3EC-tTsUyZogppY5~tU0ntdxjPy3ORMueMyWh9g5BXTim9n2HCGJ~gNoaXXUKhBAob2bmQJSMJn2UaqBTnu2rMo3YZRJiRsHXe5bgxjWVPoUNRDDxX7sq3I-b1o1s9~dPIv2fkaNqhsSy3B-IH-kroiqjA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
