Key Points
HDAC inhibition reduced proinflammatory cytokines and increased regulatory T-cell number and function after allo-HCT.
HDAC inhibition enhanced signal transducer and activator of transcription 3 acetylation and induced indoleamine-2,3-dioxygenase after allo-HCT.
Abstract
We examined immunological responses in patients receiving histone deacetylase (HDAC) inhibition (vorinostat) for graft-versus-host disease prophylaxis after allogeneic hematopoietic cell transplant. Vorinostat treatment increased histone acetylation in peripheral blood mononuclear cells (PBMCs) from treated patients, confirming target HDAC inhibition. HDAC inhibition reduced proinflammatory cytokine levels in plasma and from PBMCs and decreased ex vivo responses of PBMCs to proinflammatory TLR-4 stimuli, but did not alter the number or response of conventional T cells to nonspecific stimuli. However, the numbers of regulatory T cells (Tregs) were increased, which revealed greater demethylation of the Foxp3 T regulatory-specific demethylation region. Vorinostat-treated patients showed increased expression of CD45RA and CD31 on Tregs, and these Tregs demonstrated greater suppression on a per cell basis. Consistent with preclinical findings, HDAC inhibition also increased signal transducer and activator of transcription 3 acetylation and induced indoleamine-2,3-dioxygenase. Our data demonstrate that HDAC inhibition reduces inflammatory responses of PBMC but enhances Tregs after allo-HCT.
Introduction
Acute graft-versus-host disease (GVHD) remains a major contributor of nonrelapse mortality after allogeneic hematopoietic cell transplant (allo-HCT).1 Its pathogenesis involves a complex network of interactions among alloreactive T cells, antigen-presenting cells (APCs), proinflammatory cytokines, and effector cells, leading to target organ injury in the host.2 In experimental models of allo-HCT, a histone deacetylase (HDAC) inhibitor (vorinostat) reduced proinflammatory cytokines3 through the induction of indoleamine-2,3-dioxygenase (IDO) in a signal transducer and activator of transcription 3 (STAT-3)-dependent manner4,5 and increased regulatory T cells (Tregs)6 to attenuate GVHD. On the basis of these experimental observations, we recently performed a clinical trial of HDAC inhibition after allo-HCT that demonstrated significantly decreased acute GVHD without an increase in relapse.7 However, whether HDAC inhibition had a similar immunologic effect on inflammatory cell and Treg responses in humans is not known.8 In the present study, we explored the role of HDAC inhibition on inflammation, conventional T cells (Tconvs), and Tregs in patients from the clinical trial7 of vorinostat after allogeneic transplant.
Study design
Study cohort and sample collection
Laboratory studies were conducted in 50 patients who underwent a clinical trial.7 Oral vorinostat was administered 10 days before the stem cell infusion and continued until day 100. Study patients were compared with patients who were similarly transplanted and given standard-of-care treatment but did not receive vorinostat (control cohort). Samples were collected under informed consents and institutional review board-approved research protocols.7 Research was conducted in accordance with the Declaration of Helsinki. No differences in clinical characteristics were observed between study participants and the control cohort (supplemental Table 1, available on the Blood Web site).
Immunoblotting
Histones and STAT-3 in peripheral blood mononuclear cells (PBMCs) were assessed by western blot, as previously described,7 using antibodies listed in the supplemental Methods.
Cytokine analyses
Cytokine production was determined in plasma and PBMC supernatant samples by ELISA and in CD11c+ PBMCs by intracellular staining, as previously described.7
Phenotype of T lymphocytes
Routine absolute lymphocyte counts were performed in the Clinical Hematology Laboratory. To enumerate CD4+, CD8+, and Treg cells, PBMCs were stained using fluorochrome-conjugated antihuman monoclonal antibodies detailed in the supplemental Methods. To detect recent thymic emigrants (RTEs), PBMCs were identified using anti-human CD4, CD25, CD31, and CD45RA.9
RNA isolation and real-time-polymerase chain reaction
Total cellular RNA was isolated from PBMCs, reverse-transcribed, and used for real-time polymerase chain reaction analysis of Foxp3 and IDO, using previously described methods and primer pairs.7
Treg-specific-demethylation region demethylation assay
CD4+CD25+ Tregs and CD4+CD25− Tconvs were sorted from PBMCs using the Regulatory T Cell Kit (Miltenyi Bioscience, Bergisch Gladbach, Germany). Genomic DNA was isolated and Treg-specific-demethylation region (TSDR) analysis performed as previously described.9,10 Polymerase chain reaction conditions and specific primers pairs are listed in the supplemental Methods.
Treg suppression assay
Tregs and Tconvs were isolated, as described earlier. Tregs were mixed at ratios of 1:4 and 1:8 with Tconvs and incubated with the presence of anti-CD3/anti-CD28 coated beads (Life Technologies, Grand Island, NY) for 72 hours. 3H-thymidine (1 μCi/well; NEN Life Sciences Products, Zaventem, Belgium) was incorporated by proliferating cells for the last 8 hours of incubation and was measured using a TopCount NXT Microplate Scintillation Counter (Perkin Elmer, Waltham, MA).
Statistical analysis
Samples from the study and control cohorts were compared using the Mann-Whitney nonparametric test. Comparison of paired samples from the same patient was made using the Wilcoxon matched-pairs signed rank nonparametric test. These statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software, Inc, La Jolla, CA). A 2-sided P-value of less than .05 indicated statistical significance.
Results and discussion
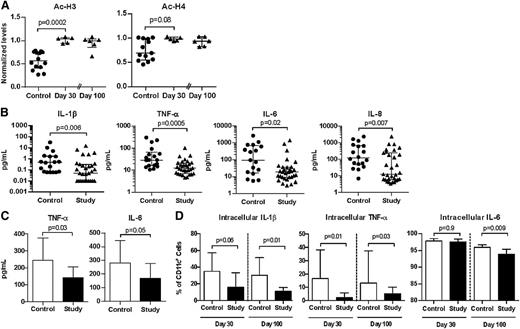
Administration of vorinostat increased H3 and H4 acetylation, confirming HDAC inhibition,8,11 and these increases had prolonged effects without significant decline 100 days after transplant (Figure 1A; supplemental Figure 1). Because HDAC inhibition mitigated inflammation and reduced GVHD in preclinical models,1-4,8 we explored the effect of vorinostat on inflammatory cell responses. Similar to preclinical observations, vorinostat-treated patients experienced significant reductions in the plasma levels of proinflammatory cytokines such as IL-1β, TNF-α, IL-6, and IL-8 14 days after transplant (Figure 1B). Interferon γ, IL-17, and IL-2 cytokines were also evaluated at this point. Although IL-17 was significantly reduced, interferon γ and IL-17 were not different between the study and control cohorts (supplemental Figure 2). We measured plasma cytokines 30 days after transplant and found significant reductions in TNF receptor-1 and IL-8 levels (supplemental Figure 3). Although plasma IL-6 levels were reduced in vorinostat-treated patients, statistical significance was not reached. IL-1β was also unchanged at day 30 between the 2 cohorts (supplemental Figure 3). Furthermore, we measured plasma biomarkers of GVHD 14 and 30 days posttransplant,12,13 such as regenerating islet-derived 3-α, suppressor of tumorigenicity 2, IL-2Rα, hepatocyte growth factor, and Elafin (supplemental Table 2). We did not find a clear correlation with clinical outcomes, which we speculate may have been a result of the conditioning regimen (reduced intensity) and the small number of patients who developed GVHD in our study.7
HDAC inhibition modulated histone acetylation and proinflammatory cytokine production after human allo-HCT. Triangles and black bars denote patients in the study who received vorinostat. Circles and open bars denote control patients who did not receive vorinostat. Median values ± interquartile range are plotted. Each data point in a dot plot represents a single patient. All plots include data from at least 2 independent experiments. (A) Levels of acetylated (Ac-) H3 and H4 30 and 100 days after allo-HCT. Levels in each patient normalized to β-actin. (B) Levels of interleukin 1β (IL-1β), tumor necrosis factor α (TNF-α), IL-6, and IL-8 in the plasma of study patients and control patients 14 days after allo-HCT. Control, n = 18; study, n = 31. (C) TNF-α and IL-6 production by PBMC after ex vivo stimulation with lipopolysaccharide (500 ng/mL) for 16 to 24 hours, 30 days after allo-HCT. Control, n = 12; study, n = 14. (D) Intracellular staining of IL1-β, TNF-α, and IL-6 in CD11c+ PBMCs of study and control patients 30 and 100 days after allo-HCT, after ex vivo stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin. Day 30: control, n = 6; study, n = 10. Day 100: control, n = 6; study n = 6.
HDAC inhibition modulated histone acetylation and proinflammatory cytokine production after human allo-HCT. Triangles and black bars denote patients in the study who received vorinostat. Circles and open bars denote control patients who did not receive vorinostat. Median values ± interquartile range are plotted. Each data point in a dot plot represents a single patient. All plots include data from at least 2 independent experiments. (A) Levels of acetylated (Ac-) H3 and H4 30 and 100 days after allo-HCT. Levels in each patient normalized to β-actin. (B) Levels of interleukin 1β (IL-1β), tumor necrosis factor α (TNF-α), IL-6, and IL-8 in the plasma of study patients and control patients 14 days after allo-HCT. Control, n = 18; study, n = 31. (C) TNF-α and IL-6 production by PBMC after ex vivo stimulation with lipopolysaccharide (500 ng/mL) for 16 to 24 hours, 30 days after allo-HCT. Control, n = 12; study, n = 14. (D) Intracellular staining of IL1-β, TNF-α, and IL-6 in CD11c+ PBMCs of study and control patients 30 and 100 days after allo-HCT, after ex vivo stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin. Day 30: control, n = 6; study, n = 10. Day 100: control, n = 6; study n = 6.
HDAC inhibition also reduced the release of proinflammatory cytokines by PBMCs ex vivo in response to lipopolysaccharide (Figure 1C) and by CD11c+ after PMA/ionomycin stimulation of bulk PBMCs (Figure 1D). These observations of reduced proinflammatory cytokines in the presence of HDAC inhibition are also consistent with other studies.14,15 Importantly, we did not observe significant differences in percentages of CD19+, CD14+, or CD11c+ cells in the PBMCs of study patients compared with controls (supplemental Table 3), nor did the percentage of CD11c+ PBMC differ between groups after PMA/ionomycin stimulation (data not shown). In contrast to preclinical findings,3 expression of MHC class II or costimulatory (CD86) molecules by CD11c+ PBMC were not changed in patients treated with vorinostat on either day 30 (supplemental Table 3) or 100 days posttransplant (data not shown).
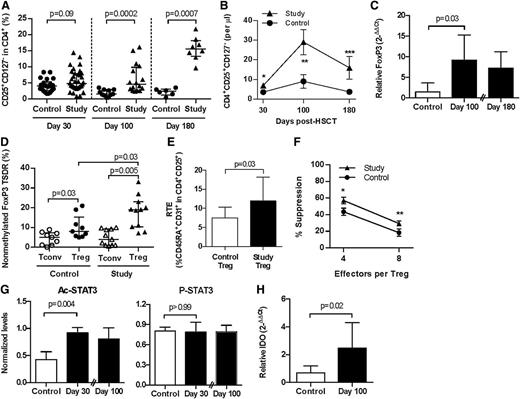
Patients with GVHD have reduced frequencies of Tregs.16-18 In murine models, Tregs attenuate GVHD,19 and HDAC inhibition increased Treg numbers and enhanced their suppressive function but did not directly alter non-Treg, that is, Tconv, responses.3,6,14,20 HDAC inhibition can also enhance suppression by human Tregs21 and promote the conversion of human T cells into Tregs in vitro.22 We therefore explored the effect of HDAC inhibition with vorinostat on both Tregs and Tconv after clinical allogeneic BMT. HDAC inhibition did not significantly alter total lymphocyte or CD4+ or CD8+ T-cell counts, nor did it alter the proliferative response of Tconv to nonspecific stimuli (supplemental Table 4; supplemental Figure 4). However, patients treated with vorinostat exhibited increased numbers of Tregs (CD4+CD25+CD127−) in the peripheral circulation (Figure 2A-B; supplemental Figure 5). Consistent with this increase, Foxp3 mRNA expression was increased in PBMC 30 days after transplant,7 which remained significantly higher 100 days posttransplant (Figure 2C). Foxp3 expression was verified on a per cell basis by flow cytometry, where more than 80% of CD4+CD25+CD127− cells were Foxp3+ (data not shown).
Patients treated with vorinostat have increased Tregs with greater suppressive function and increased acetylation of STAT-3 and IDO expression after allo-HCT. Triangles and black bars denote patients who received vorinostat. Circles and open bars denote control patients. All plots include, or are representative of, data from at least 2 independent experiments. Mean values ± SEM are plotted in B and F. Median values ± interquartile range are plotted in all other panels. (A-B) CD25+CD127− cells within the CD4+ population of PBMCs 30, 100, and 180 days after allo-HCT. Day 30: control, n = 22; study, n = 36. Day 100: control, n = 11; study, n = 16. Day 180: control, n = 6; study, n = 8. *Control vs study, P = .01; **P = .007; ***P = .06. (C) Foxp3 expression in PBMCs 100 and 180 days after allo-HCT. Data are expressed relative to glyceraldehyde-3-phosphate dehydrogenase copy number. Day 100: control, n = 6; study, n = 6. Day 180: study, n = 9. (D) Methylation of the Foxp3 TSDR in purified CD4+CD25− conventional (Tconv, open icons) and CD4+CD25+ regulatory (Treg, black icons) T cells 100 days after allo-HCT. Control, n = 9; study, n = 11. (E) CD4+CD25+CD45RA+CD31+ RTE 100 days after allo-HCT. Control, n = 5; study, n = 6. (F) Suppression of effector Tconv proliferation to anti-CD3 and anti-CD28 stimulation by autologous Tregs 100 days after allo-HCT. Control, n = 8; study, n = 10. *P = .04; **P = .07. (G) Levels of Ac- and phosphorylated (P)-STAT-3 30 and 100 days after allo-HCT. Ac- and P-STAT-3 levels normalized to total STAT-3. Day 30, control, n = 3 to 6; study, n = 5; Day 100: study, n = 6. (H) IDO messenger RNA (mRNA) expression 100 days after allo-HCT. Values are expressed relative to glyceraldehyde-3-phosphate dehydrogenase mRNA copy number. Control, n = 6; study, n = 6.
Patients treated with vorinostat have increased Tregs with greater suppressive function and increased acetylation of STAT-3 and IDO expression after allo-HCT. Triangles and black bars denote patients who received vorinostat. Circles and open bars denote control patients. All plots include, or are representative of, data from at least 2 independent experiments. Mean values ± SEM are plotted in B and F. Median values ± interquartile range are plotted in all other panels. (A-B) CD25+CD127− cells within the CD4+ population of PBMCs 30, 100, and 180 days after allo-HCT. Day 30: control, n = 22; study, n = 36. Day 100: control, n = 11; study, n = 16. Day 180: control, n = 6; study, n = 8. *Control vs study, P = .01; **P = .007; ***P = .06. (C) Foxp3 expression in PBMCs 100 and 180 days after allo-HCT. Data are expressed relative to glyceraldehyde-3-phosphate dehydrogenase copy number. Day 100: control, n = 6; study, n = 6. Day 180: study, n = 9. (D) Methylation of the Foxp3 TSDR in purified CD4+CD25− conventional (Tconv, open icons) and CD4+CD25+ regulatory (Treg, black icons) T cells 100 days after allo-HCT. Control, n = 9; study, n = 11. (E) CD4+CD25+CD45RA+CD31+ RTE 100 days after allo-HCT. Control, n = 5; study, n = 6. (F) Suppression of effector Tconv proliferation to anti-CD3 and anti-CD28 stimulation by autologous Tregs 100 days after allo-HCT. Control, n = 8; study, n = 10. *P = .04; **P = .07. (G) Levels of Ac- and phosphorylated (P)-STAT-3 30 and 100 days after allo-HCT. Ac- and P-STAT-3 levels normalized to total STAT-3. Day 30, control, n = 3 to 6; study, n = 5; Day 100: study, n = 6. (H) IDO messenger RNA (mRNA) expression 100 days after allo-HCT. Values are expressed relative to glyceraldehyde-3-phosphate dehydrogenase mRNA copy number. Control, n = 6; study, n = 6.
Foxp3 is a critical regulator of Treg development and function23 and is subject to epigenetic regulation through methylation of cytosine guanine dinucleotide islands within the TSDR.24 Thymus-derived Tregs with stable expression of Foxp3 show TSDR demethylation, whereas recently activated conventional human T cells and Tregs induced in the periphery with transient Foxp3 expression show methylated TSDR.25 HDAC inhibition with vorinostat increased demethylation of the Foxp3 TSDR in CD4+CD25+ T cells 100 days posttransplant (Figure 2D). Consistent with increased TSDR demethylation, HDAC inhibition also increased the numbers of CD45RA+CD31+ Treg RTEs (Figure 2E; supplemental Figure 6), whereas Tconv RTEs (CD4+CD25−CD45RA+CD31+) were not changed (P = .42; data not shown). Preliminary analysis also demonstrated a trend toward increased TRECs in CD4+CD25+ Treg (supplemental Figure 7). Thus, although we cannot rule out the possibility that vorinostat also induced Tregs in the periphery, the data suggest that at least a portion of the increased Tregs observed in study patients were natural Tregs derived from the thymus. We next determined the functionality of these Tregs. Tregs isolated from vorinostat-treated patients suppressed effector T-cell proliferation more effectively on a per cell basis (Figure 2F). However, antigen-specific functional studies (eg, recovery of CMV- or EBV-specific T cells) were not performed.
HDAC inhibition in mice increased acetylation of the nonhistone protein STAT-3,5 which is critical for the induction of IDO in APCs,4 reduction in inflammation, and the regulation of acute GVHD.4 Because vorinostat reduced acute GVHD7 and mitigated inflammatory cytokines, we next analyzed STAT-3 acetylation and IDO induction in these patients. Concordantly, levels of Ac-STAT-3 protein were significantly increased with HDAC inhibition 30 days posttransplant, and levels remained high at day 100 (Figure 2G). P-STAT-3 levels were unchanged (Figure 2G), consistent with a previous study.26 IDO is an intracellular enzyme that degrades tryptophan, suppresses APC function, and induces T-cell anergy.27 IDO mRNA was also induced with HDAC inhibition on day 30 posttransplant (P < .0001)7 and remained high at day 100 (Figure 2H).
Our data in murine models of HCT have shown that vorinostat modulates the production of inflammatory cytokines and induces IDO expression by host APCs to modulate GVHD after allo-HCT.3-5 Additional murine data by others indicate that HDAC inhibitors promote the generation and function of regulatory T cells.6 Ex vivo studies using human cells have confirmed the abilities of HDAC inhibitors to modulate both APCs and Tregs.20,22 Thus, collective findings suggest that HDAC inhibition can have potent and distinct direct regulatory effects on various immune cell populations. Although the mouse continues to be an important in vivo model for human immunology, we understand that potential limitations of extrapolating data from mice to humans exist.28 It is likely significant that human recipients of allo-HCT receive treatment with vorinostat concomitant with immunosuppressive therapy (tacrolimus and mycophenolate mofetil). It is possible that our inability to find quantitative reproducible differences in HLA and costimulatory molecule expression in human PBMC samples is reflective of direct effects of immunosuppression on APCs. Additional ex vivo analysis will be required to investigate this possibility. Nonetheless, the findings here demonstrate that HDAC inhibition with vorinostat after allo-HCT in humans regulated inflammation and enhanced Tregs. They further suggest that regulation of inflammation and increased Tregs might have been crucial for the ability to reduce clinical GVHD in the first-in-human clinical trial,7 and collectively, suggest that HDAC inhibition has immune-regulatory effects in humans. Future studies are needed to further delineate which immune cells are affected by HDAC inhibition after allo-HCT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Leukemia and Lymphoma Society, the National Institutes of Health (National Cancer Institute grant R01CA143379 [P.R.], and National Institute of Allergy and Infectious Diseases grants 1K23AI091623 [S.W.C.], and AI-15614 [C.A.D.]), the A. Alfred Taubman Institute Emerging Scholar Program, and the Michigan Institute for Clinical and Health Research (UL1TR00043).
Authorship
Contribution: S.W.C. and P.R. designed the clinical trial and the laboratory experiments, reviewed clinical and laboratory data, and wrote the manuscript; E.G. reviewed laboratory data, designed and analyzed laboratory experiments, and wrote the manuscript; G.H. performed and analyzed laboratory experiments and reviewed and helped write the manuscript; Y. Sun designed and performed experiments related to immunoblotting and PCRs; Y. Song reviewed laboratory data and prepared figures for publication and reviewed the manuscript; I.T. performed and analyzed laboratory experiments; C.A.D. analyzed data and reviewed and helped write the manuscript; K.O.-W. and J.W. contributed to the experiments and reviewed and helped write the manuscript; and all authors vouch for the accuracy and completeness of the data and for the analyses.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pavan Reddy, Blood and Marrow Transplantation Program, University of Michigan, 1500 E Medical Center Dr, 3312 Cancer Center, Ann Arbor, MI, 48109-5942; e-mail: reddypr@umich.edu.