Abstract
B cell precursor acute lymphoblastic leukemia (BCP-ALL) is one of the most common malignancies in children. In the period 1991-2013, the Dutch Childhood Oncology Group (DCOG) has completed three treatment trials for childhood ALL: ALL8, 9 and 10, each protocol with stratifications into risk-groups (details: www.skion.nl). Although the cure rates increased in these subsequent trials, relapses still occurred in a significant number of children. Since consecutive upfront treatment protocols usually change at multiple levels, genomic alterations that are associated with relapse may also be variable, which could provide insight into the biology underlying therapy failure and relapse. In this study, we characterized the genetic architecture of relapsed BCP-ALL patients within the context of these three Dutch upfront protocols.
We identified 3 patient groups based on upfront treatment as follows: Group-1: patients treated upfront with high-amounts of corticosteroids (CS) and relatively mild additional chemotherapy (ALL9 NHR/HR); Group-2: patients treated with high-amounts of CS and intensive additional chemotherapy (ALL10 MR); Group-3: patients treated with low-amounts of CS and moderately-intensive additional chemotherapy (ALL8 SR/MR, ALL10 SR). The number of high-risk patients that relapsed after ALL8 HR and ALL10 HR chemotherapy courses was too low to be included for analysis.
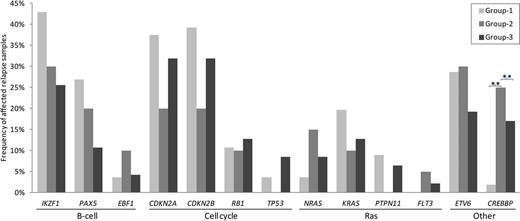
We determined, at relapse, the presence of copy number alterations and sequence mutations in 21 recurrently affected genes involved in B-cell development, cell cycle regulation and RAS signaling, in 123 patients that relapsed after treatment in group-1 (n=56), group-2 (n=20) and group-3 (n=47). The number of CREBBP mutations in patients that relapsed after treatment according to group-1 (ALL9) was significantly lower compared to the other two groups, whereas B-cell development alterations were most common in patients that relapsed after treatment according to group-1, mainly due to a higher number of IKZF1 alterations (Figure 1).
The high number of relapsed patients with leukemic clones carrying IKZF1 alterations in patients treated with high-amounts of CS and relatively mild additional chemotherapy is in line with our recent finding that IKZF1 is a key determinant of GC-induced apoptosis in normal and leukemic B-cells, and that loss of IKZF1 function confers resistance to dexamethasone, the major treatment component in group-1 (Marke et al., submitted). Additionally, in the group-2 patients treated with high-amounts of CS and highly intensive additional chemotherapy, a lower percentage IKZF1-deleted clones was detected at relapse, indicating that more GC-resistant, IKZF1-deleted clones are killed by the intense chemotherapy given in addition to CS in group-2 patients. Similarly, in the group-3 patients relapsing after treatment with lower amounts of CS and moderately-intensive additional chemotherapy, the percentage of surviving IKZF1-deleted clones was lower than in patients treated with high-amounts of CS.
Taken together, our data indicate that the genetic architecture of relapsed BCP-ALL patients depends on the upfront treatment and, in addition, that the poor-prognostic feature of IKZF1-deletions may be more prominent in upfront treatment with high-amounts of CS and relatively mild additional chemotherapy.
The frequency of genetic alterations in studied genes in patients that relapsed after treatment according to group-1, 2 and 3. Genes were grouped by their corresponding pathways. Group-1: patients treated upfront with high-amounts of CS and relatively mild additional chemotherapy (ALL9 NHR/HR); Group-2: patients treated with high-amounts of CS and intensive additional chemotherapy (ALL10 MR); Group-3: patients treated with low-amounts of CS and moderately-intensive additional chemotherapy (ALL8 SR/MR, ALL10 SR). Asterisk showed significant difference between upfront treatment groups, **p<0.001.
The frequency of genetic alterations in studied genes in patients that relapsed after treatment according to group-1, 2 and 3. Genes were grouped by their corresponding pathways. Group-1: patients treated upfront with high-amounts of CS and relatively mild additional chemotherapy (ALL9 NHR/HR); Group-2: patients treated with high-amounts of CS and intensive additional chemotherapy (ALL10 MR); Group-3: patients treated with low-amounts of CS and moderately-intensive additional chemotherapy (ALL8 SR/MR, ALL10 SR). Asterisk showed significant difference between upfront treatment groups, **p<0.001.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.