Abstract
Context: Response to treatment in CMML is difficult to evaluate. Most clinical trials so far included CMML patients (pts) together with MDS pts and assessed response with MDS IWG 2006 criteria (Cheson, Blood 2006), which poorly predict overall survival (OS) with HMA. Recently, an International Consortium has proposed MDS/MPN response criteria (IC-MDS/MPN), considering reduction in blasts (marrow response), but also correction of cytopenias or of myeloproliferation (clinical benefit; Savona, Blood 2015). These new criteria have never been compared to IWG 2006 and their prognostic relevance is unknown.
Methods: We analyzed a retrospective cohort of advanced CMML pts treated ith AZA (within EMA label) or DAC (in GFM trials) in GFM centers and Dresden. All analyses were stratified on HMA. Agreement between criteria was assessed by Cohen's kappa, a measure of reproducibility ranging from 0 (no concordance) to 1 (perfect concordance). Survival analysis was censored at transplant and Cox models were performed by Mantel-Byar method, considering achievement of response at first assessment as a time-dependent variable.
Results: The cohort included 80 pts (M/F: 56/24) with a median age of 70y (range: 41-91). HMA was AZA and DAC in 49 and 31 pts, respectively (resp.). Median interval between diagnosis and onset of HMA was 4.3 months. At onset of HMA, WHO diagnosis was CMML-1 and CMML-2 in 56% and 44%, resp. Splenomegaly was present in 40%. Median WBC, Hb and Plt counts were 14.7 10^9/L, 9.6 g/dL and 103 10^9/L, resp.; 48% pts were RBC-TD. Cytogenetic risk according to CPSS (Such, Haematologica 2011) was fav/int/low in 63/13/24% resp. CPSS risk category (Such, Blood 2013) was low/int-1/int-2/high in 10/21/54/15%, resp. ASXL1 was mutated in 43% of pts and GFM risk (Itzykson, JCO 2013) was fav/int/low in 29/36/35%, resp.
DAC and AZA were administered at standard regimens (20 mg/m2/d x5d/28d; 75 mg/m2/d x7d/28d), for a median of 9 and 7 cycles in DAC and AZA pts, resp. (p=.96). AZA regimen was intensified or reduced in 2 and 5 pts, resp. HY was administered concomitantly for the first cycles in 11%. Median follow-up was 59 months, during which 12 pts were transplanted. Median OS and AML-free survival (AMLFS) were 25.7 and 21.0 months, resp.
Initial response was assessed after a median of 4 cycles (AZA/DAC: 5/3, p<.0001); overall response rate (ORR) according to IWG 2006 was 45%, including 14% CR, 11% marrow CR (mCR), no PR, 20% stable disease with HI, while the remaining 55% had stable (SD: 21%) or progressive (PD: 34%), disease. ORR according to CI-MDS/MPN was 65%, including CR in 6%, optimal (OMR) and partial (PMR) marrow response in 44 and 4% resp. and clinical benefit (CB) in 11%.
Best IWG 2006 and IC-MDS/MPN response were achieved after a median of 5 (range: 1-18) cycles, without difference between HMA. Improvement from initial assessment to best response was noted in 12 and 11 pts with IWG 2006 and IC-MDS/MPN criteria, resp., leading to ORR of 56% (CR 21%, mCR 11%, HI 24%) and 71% (CR 12%, OMR 28%, PMR 1%, CB 30%), resp. PR was not observed with either set of criteria. A moderate agreement was found between these two sets of criteria at initial evaluation (agreement in 78% cases, Cohen's kappa: .56), with better agreement for best response (85% cases, kappa: .68).
Responses according to IC-MDS/MPN criteria were more prolonged than those defined by IWG 2006 (median duration: 19.3 vs 10.8 months, resp. p=.0004), possibly because the former, but not the latter, require additional criteria in addition to blast increase to define progression.
CPSS risk category did not predict IWG 2006 or IC-MDS/MPN response (p>0.2), but higher GFM risk tended to predict lower rates of IWG 2006 (p=.06) or IC-MDS/MPN (p=.15) response.
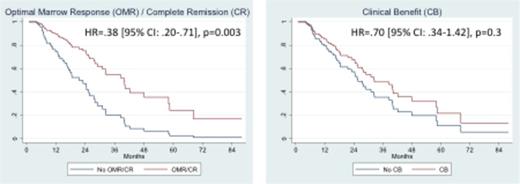
Achievement of IWG 2006 (HR=.45, p=.011) or of IC-MDS/MPN (HR= .33, p=.001) response both lead to prolonged OS. Further dissecting IC-MDS/MPN response subtypes, a significant OS benefit was found in patients achieving OMR or CR (p=.003, Figure), but not in those achieving only CB (p=.31) or partial marrow response (p=.76). Similar findings were made for AMLFS.
Conclusion: Compared to IWG 2006, IC-MDS/MPN responses to HMA in CMML are more frequent and more prolonged. CPSS and GFM risk scores do not predict response. Optimal marrow response (OMR, reduction of bone marrow blast < 5%) at first assessment predicts longer OS and AML-free survival and could be a relevant short-term endpoint for future clinical trials of HMA in CMML.
Off Label Use: Decitabine (use off label in CMML). Platzbecker:AMGEN: Honoraria; NOVARTIS: Honoraria; CELGENE: Honoraria. Park:Hospira: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding. Vey:Celgene: Honoraria; Roche: Honoraria; Janssen: Honoraria. De Botton:Agios Pharmaceuticals: Research Funding. Fenaux:NOVARTIS: Honoraria, Research Funding; AMGEN: Honoraria, Research Funding; JANSSEN: Honoraria, Research Funding; CELGENE: Honoraria, Research Funding. Itzykson:Oncoethix: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.