Abstract
Introduction: Intensive induction-consolidation chemotherapy achieves high rates of complete response (CR) in 90% of patients with newly diagnosed acute lymphoblastic leukemia (ALL). However, almost a half of the patients relapse and their outcome after frontline chemotherapy failure is essentially poor.
Methods: We retrospectively reviewed 463 patients with newly diagnosed Philadelphia-negative ALL from June 2002 to February 2015 at our institution. Overall survival was defined as the time interval from the date of relapse to the date of death. Kaplan-Meier method was used for survival analysis.
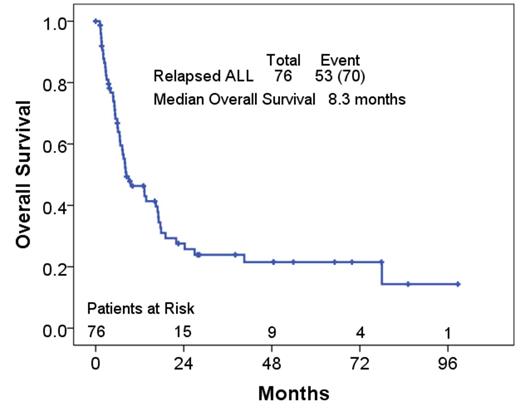
Results: Of the 463 patients, 155 (33%) relapsed. Data on salvage therapy and long term follow-up was available in 76 patients (17%). The median time to relapse was 15 months (range, 1-111 months). The median number of salvage regimens administered was 1 (range, 1-7). Overall, 76 patients received at least 1 salvage therapy. Thirty (39%) patients received at least 2 salvage regimens and 18 (24%) received 3 or more salvage regimens. Baseline patient characteristics are summarized in the table 1. Median follow-up after frontline therapy failure was 16 months. The median survival after relapse was 8.3 months with the 1- year and 2-year survival rates being 46 % and 28% respectively.
Salvage 1 included augmented HCVAD [n=13; 7/13 responses (6 CR, 1 CRp) for a median of 6 months], asparaginase based therapies [n=6; 2/6 response (2 CR) for a median of 2 months], monoclonal antibodies (MAB), blinatumomab, inotuzumab ozogamicin [n=19; 11/19 responses (6 CR, 5 CRp) for a median of 7 months], HCVAD + anti-CD20 antibody [n=11; 8/11 responses (5 CR, 3 CRp) for a median of 6 months], Miscellaneous [n=22; 2/22 responses (2 CR)] and HCVAD [n=5; 1/5 response (1 CR) for 1 month]. The overall response rate to Salvage 1 was 41% (22 CR, 9 CRp) for a median of 6 months. Nineteen (25%) patients received subsequent allogeneic stem cell transplantation (ASCT); 11 of them are alive with a median of 2 years with 7 of them in CR.
Thirty patients received a second salvage regimen; the most commonly used one consisted of MAB (blinatumomab; inotuzumomab ozogamicin) [n=8; 4/8 responses (2 CR, 2 CRp) for a median of 2.5 months]. The overall response rate to salvage 2 was 30% (6 CR, 3 CRp) for a median of 3 months.
At the last follow-up, overall 23 patients remained alive, 9 of them in CR.
Conclusions: Outcome of patients with Philadelphia-negative ALL post frontline therapies failure is poor with a median survival of only 8.3 months. Though some salvage therapies can induce remissions, response durations are limited. Stem cell transplant after remission offers a potential of long term cure. These patients should be referred to clinical trials.
Baseline characteristics and outcome of adults with relapsed B cell ALL (Ph -) who received salvage chemotherapy:
| . | N (%)/ Median [range] N= 76 . |
|---|---|
| Age (years) | 36 (18-86) |
| Age ³ 60 | 15 (20) |
| Male | 46 (61) |
| PS 2-3 | 9 (12) |
| WBC at diagnosis (x 109/L) | 7.2 [1-602] |
| CD20 positivity at diagnosis | 24 (32) |
| Cytogenetic Abnormality | |
| Diploid | 22 (29) |
| Hypodiploid | 8 (11) |
| Hyperdiploid | 12 (16) |
| t(4;11) | 5 (7) |
| Miscellaneous | 28 (37) |
| Type of Induction chemotherapy, No. (%) | |
| Augmented BFM | 28 (37) |
| HCVAD | 21 (28) |
| HCVAD + anti-CD20 antibody | 27 (35) |
| Overall response to frontline therapy | |
| CR | 73 (96) |
| CR without platelet count recovery | 2 (3) |
| Partial response | 1 (1) |
| Median response duration, (month) | 15[1-63] |
| Response duration <12 months | 35 (46) |
| Complete response to salvage chemotherapy | |
| S1 | 31/76 (41) |
| S2 | 9/30 (30) |
| S3 or more | 3/18 (17) |
| Allogeneic stem cell transplant | 19 (25) |
| . | N (%)/ Median [range] N= 76 . |
|---|---|
| Age (years) | 36 (18-86) |
| Age ³ 60 | 15 (20) |
| Male | 46 (61) |
| PS 2-3 | 9 (12) |
| WBC at diagnosis (x 109/L) | 7.2 [1-602] |
| CD20 positivity at diagnosis | 24 (32) |
| Cytogenetic Abnormality | |
| Diploid | 22 (29) |
| Hypodiploid | 8 (11) |
| Hyperdiploid | 12 (16) |
| t(4;11) | 5 (7) |
| Miscellaneous | 28 (37) |
| Type of Induction chemotherapy, No. (%) | |
| Augmented BFM | 28 (37) |
| HCVAD | 21 (28) |
| HCVAD + anti-CD20 antibody | 27 (35) |
| Overall response to frontline therapy | |
| CR | 73 (96) |
| CR without platelet count recovery | 2 (3) |
| Partial response | 1 (1) |
| Median response duration, (month) | 15[1-63] |
| Response duration <12 months | 35 (46) |
| Complete response to salvage chemotherapy | |
| S1 | 31/76 (41) |
| S2 | 9/30 (30) |
| S3 or more | 3/18 (17) |
| Allogeneic stem cell transplant | 19 (25) |
Overall survival
Cortes:Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; BerGenBio AS: Research Funding; BMS: Consultancy, Research Funding; Teva: Research Funding; Ariad: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Ambit: Consultancy, Research Funding; Arog: Research Funding; Celator: Research Funding; Jenssen: Consultancy. Daver:ImmunoGen: Other: clinical trial, Research Funding. Konopleva:Novartis: Research Funding; AbbVie: Research Funding; Stemline: Research Funding; Calithera: Research Funding; Threshold: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.