Abstract
Introduction: Cytogenetic abnormalities in patients (pts) with multiple myeloma (MM) are of prognostic importance and can be associated with poor outcomes (Bergsagel, Blood, 2011). The FIRST trial is a pivotal phase 3 study with the largest data set in transplant-ineligible pts with newly diagnosed MM (NDMM). This subanalysis evaluates the impact of cytogenetics on outcomes in transplant-ineligible pts with NDMM continuously treated with lenalidomide plus low-dose dexamethasone until disease progression (Rd continuous).
Methods: Transplant-ineligible pts with NDMM were randomized to 1 of 3 treatment arms: Rd continuous, Rd18 (Rd for 18 cycles [72 weeks]), or melphalan-prednisone-thalidomide (MPT; for 12 cycles [72 weeks]). Cytogenetics were assessed using fluorescence in situ hybridization. Pts were categorized into cytogenetic risk groups according to International Myeloma Working Group criteria. High-risk cytogenetics included del(17p), t(4;14), and t(14;16); all other pts were categorized as non-high risk. The primary endpoint was progression-free survival (PFS; primary comparators were Rd continuous vs MPT), and key secondary endpoints were overall survival (OS), overall response rate (ORR), and safety.
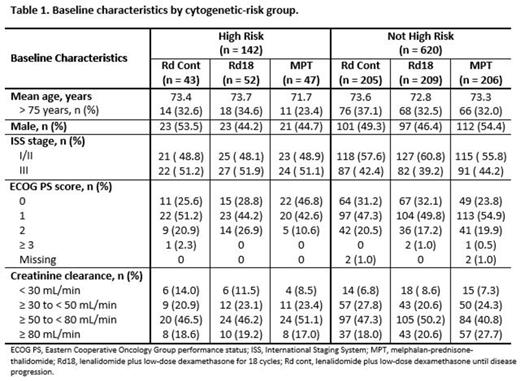
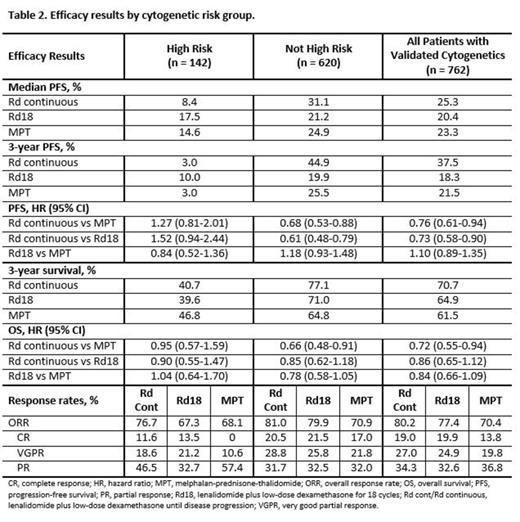
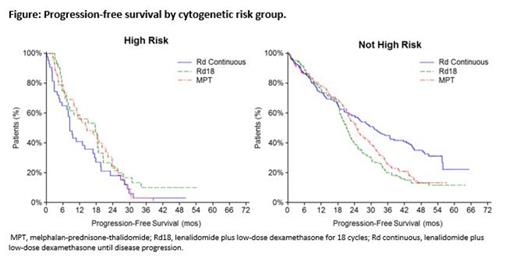
Results: A total of 762 of 1623 pts from the intent-to-treat population had validated cytogenetic profiles, with 142 pts in the high-risk group and 620 pts in the non-high-risk group. Baseline characteristics were well balanced across cytogenetic risk groups (Table 1). The median follow-up for OS was 40.2 months for the 762 pts in this analysis (data cutoff, March 03, 2014). In the non-high-risk group, median duration of treatment was 19.4 months with Rd continuous and 16.6 months with both Rd18 and MPT. In the high-risk group, median duration of treatment was 10.0 months with Rd continuous, 8.2 months with Rd18, and 12.0 months with MPT. Rd continuous treatment resulted in a 24% reduced risk of death or progression compared with MPT and an even greater 32% reduced risk in pts without high-risk cytogenetics (Table 2). In non-high-risk pts, median PFS was 31.1 months with Rd continuous compared with 21.2 and 24.9 months with Rd18 and MPT, respectively (Figure). However, in high-risk pts, the observed numerical median PFS favoring Rd18 is mainly due to small pt numbers influenced by long runners (n = 5), and the greatly overlapping 95% CIs from all 3 arms show the difference is likely to be minimal. Rd continuous treatment resulted in a 28% reduced risk of death vs MPT overall and a 34% reduced risk in pts without high-risk cytogenetics. OS was similar across treatment arms for high-risk pts. ORRs in all cytogenetic risk groups favored Rd continuous vs MPT. In pts with high-risk cytogenetics, higher-quality responses were also observed with Rd continuous vs MPT treatment. Similar results were seen with Rd continuous compared with Rd18, although OS and ORR benefits overall and in pts without high-risk cytogenetics were not as pronounced. In all 3 treatment arms, adverse events were consistent across cytogenetic risk groups.
Conclusions: Rd continuous treatment resulted in PFS and OS benefits vs MPT in pts with validated cytogenetic profiles. This was largely due to PFS and OS improvements in pts without high-risk cytogenetics. In the high-risk group, the longest PFS was observed with Rd18 treatment and OS was similar across treatment arms. Despite being on the continuous vs fixed duration treatment arm, high-risk pts on Rd continuous received a shorter duration of treatment than those on MPT, which may explain why PFS favored MPT vs Rd continuous. Higher response rates were observed with Rd continuous vs MPT, regardless of cytogenetic risk, and greater quality responses were observed in pts with high-risk cytogenetics. The safety profile of Rd continuous was manageable and consistent between cytogenetic risk groups. Results support Rd continuous as a standard treatment option for pts with NDMM who are ineligible for transplant, especially those without high-risk cytogenetics. Additional PFS and OS benefits may be achieved in pts with high-risk cytogenetics when Rd continuous is used as a backbone for combination therapy with a novel agent. Promising activity in pts with high-risk cytogenetic abnormalities has been demonstrated using this approach (Lonial et al, N Engl J Med, 2015; Stewart et al, N Engl J Med, 2015).
Hulin:Celgene Corporation: Honoraria; Janssen: Honoraria; Amgen: Honoraria; Bristol Myers Squibb: Honoraria. Dimopoulos:Celgene: Honoraria; Onyx: Honoraria; Novartis: Honoraria; Janssen-Cilag: Honoraria; Amgen: Honoraria; Janssen: Honoraria; Genesis: Honoraria. Reece:Lundbeck: Honoraria; Amgen: Honoraria; Merck: Research Funding; Bristol-Myers Squibb: Research Funding; Janssen-Cilag: Consultancy, Honoraria, Research Funding; Onyx: Consultancy; Celgene: Consultancy, Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Millennium Takeda: Research Funding; Otsuka: Research Funding. Catalano:Roche: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Celgene Corporation: Consultancy, Honoraria. Pinto:Takeda: Honoraria, Research Funding; Celgene Corporation: Honoraria; Spectrum: Honoraria. Ludwig:Takeda: Research Funding; Celgene Corporation: Honoraria, Speakers Bureau; Onyx: Honoraria, Speakers Bureau; Bristol Myers Squibb: Honoraria, Speakers Bureau; Janssen Cilag: Honoraria, Speakers Bureau. Bahlis:Celgene Corporation: Honoraria, Research Funding. Cavo:Janssen-Cilag, Celgene, Amgen, BMS: Honoraria. Moreau:Takeda: Other: Adboard; Janssen: Other: Adboard; Novartis: Other: Adboard; Amgen: Other: Adboard; Celgene: Honoraria, Other: Adboard. Qiu:Johnson & Johnson: Speakers Bureau; Celgene Corporation: Speakers Bureau; Roche: Speakers Bureau. Schots:Celgene Corporation: Research Funding. Marek:Celgene Corporation: Employment, Equity Ownership. Chen:Celgene Corporation: Employment, Equity Ownership. Yiu:Celgene Corporation: Employment, Equity Ownership. Ervin-Haynes:Celgene Corporation: Employment. Facon:Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Millenium: Membership on an entity's Board of Directors or advisory committees; Onyx: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Pierre Fabre: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract