Abstract
CD33 is variably expressed on acute myeloid leukemia (AML) blasts and is the target of gemtuzumab ozogamicin (GO). We previously demonstrated the clinical benefit of GO treatment in children with AML treated on COG AAML0531 in which patients were randomized to receive standard Medical Research Council-based chemotherapy with or without GO. We also demonstrated that CD33 expression is highly variable in pediatric AML and that children with 11q23 translocations involving the KMT2A gene, previously known as the mixed lineage leukemia gene and referred to here as MLL+, have significantly higher CD33 expression, as defined by mean fluorescent intensity (MFI) values, than patients without 11q23/MLL + leukemia (MLL-) [median CD33 MFI: MLL + 229.13 (range 6-1351) vs. MLL-129 (range 2.68-1225.87) P <0.001.] Given significantly elevated levels of CD33 expression in MLL + AML and our previous findings showing an association between high CD33 expression and improved response to GO, we evaluated MLL + AML patients treated on COG AAML0531 to determine whether GO treatment improved their clinical outcomes.
COG AAML0531 included 1022 eligible patients ages 1 month-29.99 years of which 215 harbored a 11q23/MLL rearrangement that was confirmed by central cytogenetic review (including G-banding and FISH). Analysis of overall outcomes revealed similar complete remission (CR) rates after Induction I for MLL + and MLL-patients (71% vs. 73%, P = 0.494). However, MLL + patients had lower 5-year overall survival (OS) and event-free survival (EFS) than MLL-patients (OS 58% vs. 66%, P =0.012, EFS 38% vs. 51%, P =<0.001) as well as higher rates of relapse (RR) (52% vs. 36%, P =<0.001) and lower disease-free survival (DFS) (46% vs. 58%, P =0.002).
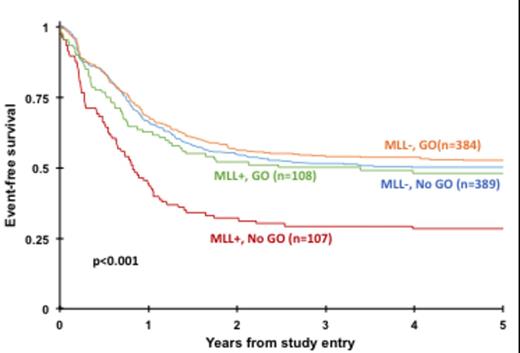
Of the 215 MLL + patients, 107 were treated with conventional chemotherapy only (No-GO) and 108 with chemotherapy and GO (GO). CD33 expression data from flow cytometry analysis were available for 170 MLL + patients. The median CD33 MFI was similar for MLL + patients on both treatment arms [No-GO: 226.5 (range 6-911), GO 237.345 (range 7.6-1351), P = 0.648]. CR rate was higher for MLL + patients treated with GO vs. No-GO (77% vs. 64%; P =0.035). Evaluation of clinical outcomes for patients in the MLL + cohort by treatment arm revealed a superior outcome for GO recipients. EFS at 5 years from study entry was 48% for patients in the GO group vs. 28% for those in the No-GO group (P =0.002) with a corresponding OS of 64% vs. 53% (P =0.053). MLL-patients had similar EFS and OS regardless of GO exposure (P =0.435 and P =0.861, respectively, Figure 1). In MLL + patients who achieved CR, GO exposure translated to lower RR (40% vs. 66% No-GO, P =0.001) and improved DFS (57% vs. 33% No-GO, P =0.002) demonstrating that MLL + patients receiving GO treatment have improved outcomes.
In COG AAML0531 a subset of patients was allocated to receive allogeneic hematopoietic stem cell transplant (HSCT) in 1st CR based on donor availability and risk status. This allowed us to evaluate the effect of HSCT in MLL+ patients in the context of GO exposure as any MLL+ patient with a matched family donor or poor induction response (>15% blasts) underwent HSCT. HSCT was conducted in 19 of 83 MLL+ patients (23%) in the GO group after one course of intensification therapy and in 11 of 73 (15%) patients in the No-GO group. Patients in the GO group who received HSCT consolidation had better outcomes than those not receiving HSCT. Specifically, MLL+ patients who received HSCT after prior treatment with GO had a RR of 28% at 5 years from HSCT compared with a RR of 73% for MLL + patients who received HSCT without GO prior (P =0.006). The corresponding DFS at 5 years from HSCT for patients in the GO and No-GO groups was 72% vs. 27% (P =0.004) respectively. These results highlight that the clinical impact of induction GO maintains clinical significance in the post-HSCT setting.
Our analysis of data from AAML0531 suggests that pediatric MLL + AML might benefit from the addition of GO to conventional chemotherapy. HSCT might further enhance GO benefit in this subset of patients. Future studies, utilizing GO or other novel CD33 targeted agents, should be considered for MLL + pediatric AML given the superior outcomes observed.
Event-free survival from study entry for 11q23/MLL + vs. MLL - patients by treatment arm (GO vs. No-GO).
Event-free survival from study entry for 11q23/MLL + vs. MLL - patients by treatment arm (GO vs. No-GO).
Aplenc:Sigma Tau: Honoraria. Loken:Hematologics Inc.: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.