Abstract
Background: Elderly patients with ALL have a significantly poor outcome. This is primarily due to poor tolerance of intensive chemotherapy. Addition of targeted non-myelosuppressive therapy to effective low-intensity chemotherapy might improve outcome. CD22 expression occurs in >90% of patients with ALL. Inotuzumab ozogamicin (IO) is a CD22 monoclonal antibody bound to a toxin, calecheamicin, and has shown single-agent activity in relapsed/refractory ALL (Kantarjian et al. Lancet Oncology 2012).
Methods: Patients ≥60 years with newly-diagnosed B-cell ALL were eligible for the chemotherapy with mini-hyper-CVD (cyclophosphamide and dexamethasone at 50% dose reduction, no anthracycline, methotrexate at 75% dose reduction, cytarabine at 0.5 g/m2 x 4 doses) in combination with IO given on Day 3 of each of the first 4 courses. Rituximab and intrathecal chemotherapy were given for first 4 courses. The first 6 pts received 1.3 mg/m2 for cycle 1 followed by 0.8 mg/m2 for subsequent cycles; Pts 7 onwards received 1.8 mg/m2 for Cycle 1 followed by 1.3 mg/m2 for subsequent cycles.
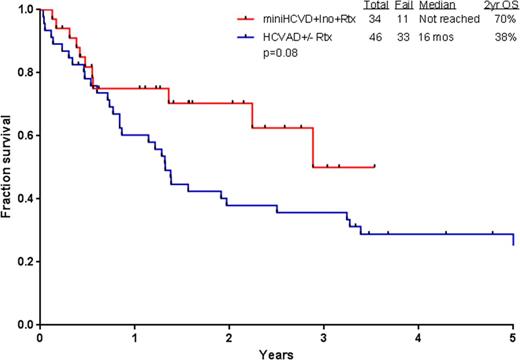
Results: Thirty-four patients (21 men, 13 women) have been treated so far. Patient characteristics and outcome are summarized in Table 1. Median age is 69 years (range 60-79). Median follow-up is 19 months. Of the 31 patients evaluable for response (3 started in CR), 30 (97%) achieved CR/CRp (25 CR, 5 CRp). All patients achieving CR have also achieved flow-cytometry MRD negative status, in 80% at the time of CR achievement. Median time to platelet and neutrophil recovery was 23 days (18-91), and 16 days (12-49) after induction, and was 22 days (14-64), and 17 days (13-49) after subsequent cycles. Grade 3-4 toxicities ≥10% included prolonged thrombocytopenia (n=27; 79%), infections during consolidation (n=25; 73%), infections during induction (n=18; 52%), hyperglycemia (n=17; 50%), hypokalemia (n=12; 35%), hyperbilirubinemia (n=8; 24%), increased ALT/AST (n=7; 21%), and hemorrhage (n=6; 18%). Of note, veno-occlusive disease (VOD) was observed in 4 patients (11%); 1 in CR and negative MRD, G2 VOD at C3D57; 1 in CR and negative MRD, G5 VOD at C2D40; 1 in CR and negative MRD, G2 VOD at D43 post matched related allogeneic stem cell transplantation with conditioning of fludarabine and busulfan after 4 cycles of mini-hyper-CVD + IO; and 1 in CR and negative MRD, G2 VOD at C2D70. At the last follow-up, 23 (68%) are alive and 22 (65%) in CR. Eleven patients (32%) died: 1 had primary refractory ALL and died after the first salvage; 2 relapsed after receiving 2 and 3 courses only due to prolonged myelossuppression and died of disease progression; and 8 died in CR from sepsis (n=4), gunshot wound (n=1), VOD (n=1), dementia (n=1), and unknown cause (n=1). Two patients (6%) received allogeneic stem cell transplantation. Four patients (12%) are currently receiving consolidation chemotherapy with a median of 5 cycles (1-8); 16 patients (47%) are receiving POMP maintenance chemotherapy. The 2-year progression-free survival and overall survival rates were 87% and 70%, respectively. The mini-hyper-CVD (n=34) appears superior to the historical HCVAD +/- rituximab (n=46) in similar patient population (2-year OS, 70% and 38%, respectively; Figure 1).
Conclusions: The combination of IO with low-intensity mini-hyper-CVD chemotherapy is safe and shows encouraging results (97% CR/CRp) in the frontline setting in older patients with ALL. These results appear to be better than those achieved with a chemotherapy alone only approach and may become the new standard of care for frontline treatment of older patients with ALL.
Patient characteristics and outcome
| Characteristic . | Median (range) / No. (%) N=34 . |
|---|---|
| Age (yrs) | 69 [60-79] |
| Male | 21 (62) |
| Performance Status (ECOG) ≥ 2 | 4 (12) |
| WBC at DX | 3.5 [0.6-111.0] |
| WBC at DX ≥ 50 | 2 (6) |
| Karyotype | |
| Diploid Complex Misc IM | 11 (32) 15 (44) 5 (15) 3 (9) |
| CD22 Positivity | 97 [72-100] |
| CD20 ≥ 20 | 20 (65) |
| Response | |
| CR | 25/31 (81) |
| CRp | 5/31 (31) |
| Cytogenetic CR | 19/19 (100) |
| Negative MRD, D21 | 20/25 (80) |
| Negative MRD, Overall | 33/33 (100) |
| ORR | 30/31 (97) |
| No response | 1/31 (3) |
| Early death | 0 |
| Characteristic . | Median (range) / No. (%) N=34 . |
|---|---|
| Age (yrs) | 69 [60-79] |
| Male | 21 (62) |
| Performance Status (ECOG) ≥ 2 | 4 (12) |
| WBC at DX | 3.5 [0.6-111.0] |
| WBC at DX ≥ 50 | 2 (6) |
| Karyotype | |
| Diploid Complex Misc IM | 11 (32) 15 (44) 5 (15) 3 (9) |
| CD22 Positivity | 97 [72-100] |
| CD20 ≥ 20 | 20 (65) |
| Response | |
| CR | 25/31 (81) |
| CRp | 5/31 (31) |
| Cytogenetic CR | 19/19 (100) |
| Negative MRD, D21 | 20/25 (80) |
| Negative MRD, Overall | 33/33 (100) |
| ORR | 30/31 (97) |
| No response | 1/31 (3) |
| Early death | 0 |
Survival with mini-hyper-CVD+IO vs hyper-CVAD +/- rituximab in frontline ALL
Survival with mini-hyper-CVD+IO vs hyper-CVAD +/- rituximab in frontline ALL
O'Brien:Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding. Konopleva:Novartis: Research Funding; AbbVie: Research Funding; Stemline: Research Funding; Calithera: Research Funding; Threshold: Research Funding. Cortes:Teva: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; BMS: Consultancy, Research Funding; ARIAD Pharmaceuticals Inc.: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract