Abstract
T follicular helper cells (TFH) interact with B cells in the germinal center to support B cell proliferation, differentiation and immunoglobulin class switch. Previous studies have demonstrated that donor B cells contribute to the immune pathology of chronic GVHD (cGVHD) and studies in murine models of cGVHD have suggested that TFH play a central role in this process. Although TFH are located primarily within germinal centers of secondary lymphoid organs, TFH can also be found in the peripheral blood and are identified as a subset of memory CD4 T cells expressing CXCR5. Circulating TFH (cTFH) share functional and phenotypic characteristics of TFH including the ability to interact with B cells and provide helper signals. To examine the potential role of cTFH in the pathogenesis of cGVHD we monitored the reconstitution of cTFH (CD4+CD45RA-CXCR5+ T cells) in fresh blood samples obtained at different times after allogeneic HSCT. Recovery of cTFH mirrored the recovery of CD4 T cells and absolute numbers of cTFH gradually increased over time, reaching a plateau at 24 months post-HSCT in the range of normal values. Within the CD4 T cell population, the frequency of cTFH was low initially, but reached normal levels 6-9 months post-HSCT.
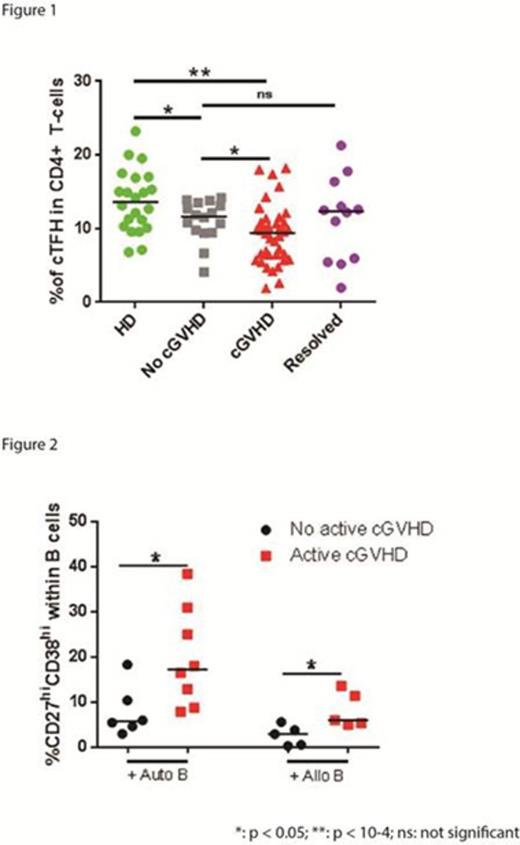
Detailed phenotypic and functional analysis of cTFH was undertaken in a cohort of 66 adult patients (>9 months post-HSCT): 16 had no cGVHD, 12 had resolved cGVHD, and 38 had active cGVHD (mild = 15, moderate = 14, severe = 9). Results were compared to 22 healthy donors (HD). Overall, the frequency of cTFH was significantly reduced in HSCT patients compared to HD (median: 9.87 vs. 13.65 % of CD4+ T-cells, respectively, p =0.0003). cTFH frequency was lower in active cGVHD compared to no cGVHD patients (median: 9.44 vs. 11.65 % of CD4+ T-cells, p = 0.029); while cTFH in patients with resolved cGVHD was similar to patients without cGVHD (Figure 1). CXCL13 chemokine facilitates T-B interaction, thus promoting the GC reaction. CXCL13 levels were increased in active cGVHD compared to no cGVHD (137.7 pg/mL vs. 33.74 pg/mL, p < 10-4) suggesting that trafficking of cTFH to lymphoid organs was promoted in cGVHD. cTFH activation results in increased surface expression of ICOS and PD1 and increased proliferation. ICOShi PD1hi cTFH were increased in active cGVHD compared to no cGVHD (2.035 % vs 1.065 %, p = 0.016) in association with high proliferative activity (Ki67) (3.38 % vs. 2.31 % respectively, p = 0.0086). Functional cTFH subsets are also characterized by expression of CXCR3 and CCR6 as follows: Th1 (CXCR3+CCR6-), Th2 (CXCR3-CCR6-), Th17 (CXCR3-CCR6+). Within these subsets, Th2 and Th17 cTFH provide greater levels of B cell help measured by in vitro co-cultureassays (plasmablast generation and IgG production). Although not statistically significant, Th1 cTFH frequency was decreased during active cGVHD (33.95 vs 37.6 for no cGVHD) and Th2 and Th17 cTFH frequencies were increased in the moderate-severe cGVHD group (31.1 and 30.5, respectively) compared to no cGVHD (27.05 and 24.45, respectively). Within active cGVHD patients, the frequencies of Th1 and Th17 were associated with clinical grade of cGVHD (Th1: 40, 34, 19, p=0.008; Th17: 21, 26, 37, p=0.02; for mild, moderate, severe, respectively). We used the ratio (Th2+Th17)/Th1 cTFH to normalize B cell help capacity, and found a higher ratio in severe cGVHD compared to no cGVHD (3.59 vs 1.36, p = 0.0006). To measure cTFH functional activity, cTFH and naive B cells were purified from patient samples by cell sorting and co-cultured for 6 days. cTFH from patients with active cGVHD induced greater plasmablast generation (CD27hi CD38hi B cells) compared to cTFH from non-active cGVHD (Fig. 2). Increased functional activity of cTFH was confirmed when patient cTFH were purified and cocultured with naive B cells from a normal allogeneic donor (Fig. 2). In patients with cGVHD, there was also a significant correlation between the proliferative activity of cTFH and the generation of CD27hi CD38hi B cells (r = 0.48, p = 0.0029).
These results indicate that active cGVHD is characterized by phenotypic and functional abnormalities of cTFH. In cGVHD, cTFH show high levels of activation, proliferation and switch toward Th2 and Th17 phenotype. These changes enhance cTFH function, promoting GC reactions and B cell differentiation. These data support the involvement of cTFH in the immune pathology of human cGVHD, suggesting the investigation of novel therapies targeting the GC reaction.
Armand:Merck: Consultancy, Research Funding; Infinity Pharmaceuticals: Consultancy; Bristol-Myers Squibb: Research Funding. Antin:Gentium S.p.A.: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.