In this issue of Blood, Tie et al report the development of a cleverly engineered, cell-based system for studying mutations in γ-glutamyl carboxylase (GGCX), the enzyme responsible for converting glutamate residues in certain proteins to γ-carboxyglutamate (Gla).1 They use this cell-based assay system to help explain the clinical manifestations of some otherwise puzzling GGCX gene mutations in humans that cause phenotypes ranging from severe bleeding to Keutel syndrome.
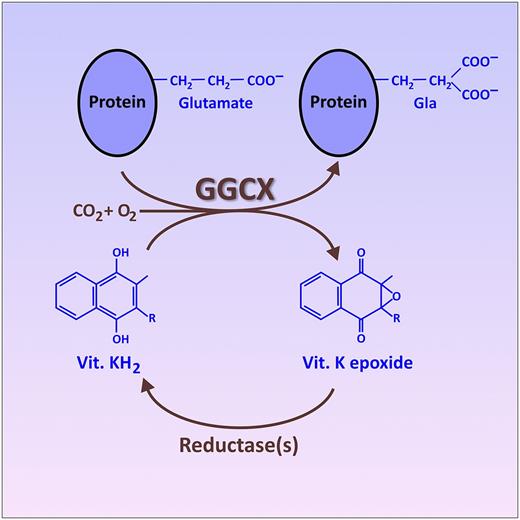
The vitamin (Vit.) K cycle, resulting in the posttranslational conversion of specific glutamate residues on certain target proteins into Gla. This conversion is catalyzed by the endoplasmic reticulum enzyme, GGCX, which utilizes the fully reduced form of vitamin K (KH2) as a cosubstrate. An oxidized form of vitamin K (epoxide), produced as a byproduct of this reaction, is recycled back into fully reduced KH2 by other enzymes (reductases). This recycling of oxidized vitamin K is the portion of the cycle inhibited by warfarin and related drugs. The figure has been adapted from Figure 2B in the article by Tie et al that begins on page 1847.
The vitamin (Vit.) K cycle, resulting in the posttranslational conversion of specific glutamate residues on certain target proteins into Gla. This conversion is catalyzed by the endoplasmic reticulum enzyme, GGCX, which utilizes the fully reduced form of vitamin K (KH2) as a cosubstrate. An oxidized form of vitamin K (epoxide), produced as a byproduct of this reaction, is recycled back into fully reduced KH2 by other enzymes (reductases). This recycling of oxidized vitamin K is the portion of the cycle inhibited by warfarin and related drugs. The figure has been adapted from Figure 2B in the article by Tie et al that begins on page 1847.
The best-known proteins that undergo the posttranslational conversion of specific glutamate residues into Gla are the vitamin K–dependent blood clotting proteins, the carboxylation of which is targeted by the class of oral anticoagulants known as “vitamin K antagonists” (including warfarin and related drugs).2 To accomplish this protein modification, the GGCX enzyme recognizes a particular propeptide sequence in the target protein and then modifies nearby glutamate residues into Gla by affixing a CO2 onto each side chain, thereby creating a second carboxylic acid functionality (see figure). GGCX uses the fully reduced form of vitamin K (KH2) as a cosubstrate, generating vitamin K epoxide as a byproduct. The oxidized vitamin K epoxide is then recycled back to KH2 via the action of a couple of reductases, only one of which is currently fully identified.2 In addition to the 7 blood-clotting proteins with Gla domains, additional Gla-containing proteins have also been discovered. Notably, this includes matrix Gla protein (MGP), which is important in regulating vascular calcification and mineralization of connective tissue.3,4
General defects in γ-carboxylation of proteins are known, resulting from mutations in the gene for GGCX or for the vitamin K epoxide reductase. Such mutations can cause combined vitamin K–dependent coagulation factor deficiency, leading to severe bleeding.5 In addition, defects in the vitamin K cycle are also associated with certain nonbleeding syndromes such as pseudoxanthoma elasticum–like syndrome.4 The standard treatment for these patients is very high doses of vitamin K, which is effective in many cases. In addition, rare mutations in MGP itself can cause Keutel syndrome, characterized by peripheral pulmonary stenosis and abnormal cartilage calcification/ossification.6 A puzzling feature of mutations in GGCX is that some result in bleeding phenotypes, whereas others result in nonbleeding syndromes, with little correlation with the apparent in vitro GGCX enzymatic activity.
Tie et al1 noted that studies of the properties of the GGCX enzyme have typically used highly artificial in vitro conditions that use artificial electron donors and lack the natural recycling of vitamin K between oxidized and reduced forms. They therefore used the powerful CRISPR/Cas9 gene–editing technology to delete the endogenous GGCX enzyme in HEK293 cells, allowing them to replace it with a recombinant gene expressing any desired GGCX mutant. By studying the degree of γ-carboxylation of artificial reporter genes that were also transfected into these cells, they were able to assess directly the effects of specific mutations in GGCX on the γ-carboxylation of blood-clotting enzymes vs MGP. The power of this cell-based assay system is that the GGCX enzyme is functioning within the natural milieu of the endoplasmic reticulum, relying on the remaining enzymes of the native vitamin K cycle to produce vitamin KH2.
Tie et al1 studied a baby with a bleeding diathesis (and features of Keutel syndrome) attributed to a specific mutation (R325Q) in GGCX. Interestingly, the patient’s bleeding diathesis responded to administration of high levels of vitamin K, but the undercarboxylation of MGP was highly resistant to vitamin K administration to this patient. They then expressed this same mutant form of GGCX in their cell-based assay system and discovered that high levels of vitamin K in cell culture corrected the γ-carboxylation of their engineered blood-clotting protein, largely to the same degree to which the patient’s blood-clotting system responded to vitamin K administration in vivo. However, MGP expressed by the cells containing the mutant GGCX failed to show any increase in γ-carboxylation in the presence of high levels of vitamin K, again recapitulating the patient’s response in terms of MGP carboxylation.
Even among the vitamin K–dependent blood-clotting proteins in this patient, the mutation in GGCX resulted in quite different responses to administration of high levels of vitamin K. The biochemical basis for this differential response is currently not known, and there are many other open questions in understanding GGCX function and substrate specificity. The availability of this facile, cell-based system for studying GGCX mutants in their native cellular environment will doubtless prove extremely useful in advancing our understanding of γ-carboxylation of vitamin K–dependent proteins.
Conflict-of-interest disclosure: The author declares no competing financial interests.