Key Points
CRISPR-Cas9–mediated GGCX knockout cell–based assay clarifies the correlation between GGCX genotypes and their clinical phenotypes.
A GGCX mutation decreases clotting factor carboxylation and abolishes MGP carboxylation, causing 2 distinct clinical phenotypes.
Abstract
Vitamin K–dependent coagulation factors deficiency is a bleeding disorder mainly associated with mutations in γ-glutamyl carboxylase (GGCX) that often has fatal outcomes. Some patients with nonbleeding syndromes linked to GGCX mutations, however, show no coagulation abnormalities. The correlation between GGCX genotypes and their clinical phenotypes has been previously unknown. Here we report the identification and characterization of novel GGCX mutations in a patient with both severe cerebral bleeding disorder and comorbid Keutel syndrome, a nonbleeding malady caused by functional defects of matrix γ-carboxyglutamate protein (MGP). To characterize GGCX mutants in a cellular milieu, we established a cell-based assay by stably expressing 2 reporter proteins (a chimeric coagulation factor and MGP) in HEK293 cells. The endogenous GGCX gene in these cells was knocked out by CRISPR-Cas9–mediated genome editing. Our results show that, compared with wild-type GGCX, the patient’s GGCX D153G mutant significantly decreased coagulation factor carboxylation and abolished MGP carboxylation at the physiological concentration of vitamin K. Higher vitamin K concentrations can restore up to 60% of coagulation factor carboxylation but do not ameliorate MGP carboxylation. These results are consistent with the clinical results obtained from the patient treated with vitamin K, suggesting that the D153G alteration in GGCX is the causative mutation for both the bleeding and nonbleeding disorders in our patient. These findings provide the first evidence of a GGCX mutation resulting in 2 distinct clinical phenotypes; the established cell-based assay provides a powerful tool for studying the clinical consequences of naturally occurring GGCX mutations in vivo.
Introduction
Vitamin K–dependent carboxylation is a posttranslational modification that converts specific glutamate (Glu) residues to γ-carboxyglutamate (Gla) residues in vitamin K–dependent proteins. It is required for the functioning of numerous vitamin K–dependent proteins involved in a broad range of biological functions, including blood coagulation. Carboxylation is catalyzed by the enzyme γ-glutamyl carboxylase (GGCX), which uses a reduced form of vitamin K (KH2) as a cofactor. Concomitant with each glutamate modification, KH2 is oxidized to vitamin K epoxide (KO). Because humans cannot synthesize vitamin K, KO must be converted back to KH2 by the enzymes KO reductase and the as-yet unknown vitamin K reductase in a pathway known as the vitamin K cycle.1
Defects of vitamin K–dependent carboxylation have long been known to cause bleeding disorders, referred to as combined vitamin K–dependent coagulation factors deficiency (VKCFD). Patients with VKCFD have decreased levels of multiple coagulation factors (II, VII, IX, and X) and of anticoagulant proteins (C and S). VKCFD commonly presents early in life with intracranial hemorrhage or umbilical stump bleeding.2-4 Diagnosis of VKCFD is based on the persistence of bleeding manifestations with prolonged prothrombin time and activated partial thromboplastin time (APTT). Two subtypes of VKCFD have been identified that are based on defects in 2 enzymes of the vitamin K cycle: VKCFD1 is associated with mutations in GGCX, whereas VKCFD2 is linked to functional deficiency of KO reductase.2,5 The mainstay of therapy for VKCFD is the administration of high-dose phylloquinone (vitamin K1), which often corrects, either completely or partially, the coagulation factors defect.6
With the discovery that carboxylated matrix Gla protein (cMGP) is a strong inhibitor of vascular calcification and connective tissue mineralization,7 vitamin K–dependent carboxylation has been implicated in cardiovascular diseases and other nonbleeding syndromes, such as pseudoxanthoma elasticum (PXE)-like syndrome.8,9 Although coagulation factors are synthesized and carboxylated in the liver, MGP is produced by chondrocytes, vascular smooth muscle cells, endothelial cells, and fibroblasts, and it undergoes local carboxylation.10 Both the hepatic and the extrahepatic carboxylations are sensitive to warfarin inhibition. Patients receiving long-term oral anticoagulants to inhibit clotting factor carboxylation display decreased MGP carboxylation and increased vascular calcification.11 Desphospho-uncarboxylated MGP (dp-ucMGP) has been reported as a biomarker for mortality risk in patients with chronic stable vascular disease.12 In addition, patients with PXE-like syndrome have increased levels of ucMGP in plasma, serum, and the dermis because of their defective vitamin K–dependent carboxylation.9,13 In rare cases, mutations in MGP cause Keutel syndrome, a disease characterized by multiple peripheral pulmonary artery stenosis, short terminal phalanges, abnormal cartilage calcification, and mid-facial hypoplasia with a depressed nasal bridge.14,15
Genetic screening of patients with vitamin K–related disorders has found more than 30 naturally occurring mutations in GGCX.16 It is not clear, however, why some mutations cause bleeding disorders while others cause nonbleeding syndromes. This lack of knowledge comes in part from the fact that our current understanding of GGCX’s function was obtained from in vitro experimentation under artificial conditions,17 which has limited usefulness in understanding the clinical consequences of GGCX mutations. In an attempt to address this issue, a GGCX-deficient mouse strain was generated by gene targeting.18 However, because of embryonic lethality, all the homozygous GGCX-deficient mice succumbed to massive intra-abdominal hemorrhage shortly after birth. Although liver-specific GGCX-deficient mice have recently been created to examine GGCX function in vivo,19 it appears that manipulating GGCX variants and GGCX substrates in mice is impracticable.
In the present study, we developed a cell-based reporter assay system that enables the functional study of GGCX in its native milieu. In this system, we coexpressed 2 vitamin K–dependent reporter proteins with distinct structures; these reporter proteins are easily measured, and reflect the efficiency of vitamin K–dependent carboxylation in vivo. To eliminate the background signal, the endogenous GGCX of the reporter cells was knocked out by CRISPR-Cas9–mediated genome editing. Using this cell-based assay, we characterized the GGCX mutations found in a patient with both severe bleeding disorder and comorbid Keutel syndrome. Results from our cell-based assay were compared with the clinical results obtained from the patient treated with K vitamins. With this novel cell-based assay and this new clinical case, we expect to clarify the correlation between the GGCX genotype and its clinical phenotype.
Methods
Research subject
The subject was selected on the basis of having the clinical phenotype of VKCFD. Written informed consent for genomic analysis and for patient sample collection was obtained from the patient’s parents at the time of sample collection. Genetic and clinical studies were conducted in accordance with the protocols approved by the Research Board of Hospital das Clínicas – Laboratório de Hemostasia e Trombose do Serviço de Hematologia do Hospital das Clínicas.
Genetic analysis
Blood samples were collected from the patient and her family members. Genomic DNAs were isolated from peripheral blood by the standard method. The GGCX mutation analysis was performed using the polymerase chain reaction (PCR) technique, followed by bidirectional automatic sequencing and reading by capillary electrophoresis.
Coagulation test and serum dp-ucMGP concentration determination
For clotting factor assays, the 1-stage APTT-based assay was used to determine FIX activity according to the manufacturer’s instructions (APTT-SP, Instrumentation Laboratory, Bedford, MA). Factor II, V, VII and X activity were determined using a 1-stage prothrombin time–based assay (PT-Fibrinogen HS plus, Instrumentation Laboratory). Protein C and protein S were determined by automated chromogenic assay and automated latex ligand immunoassay, respectively.
Circulating dp-ucMGP was quantified in citrated plasma samples using the InaKtif MGP iSYS kit (IDS, Boldon, UK), which is a precommercial dual-antibody test based on the sandwich enzyme-linked immunosorbent assay (ELISA) developed by VitaK (Maastricht University, the Netherlands).20
Establishment of cell-based carboxylation activity assay with 2 reporter proteins
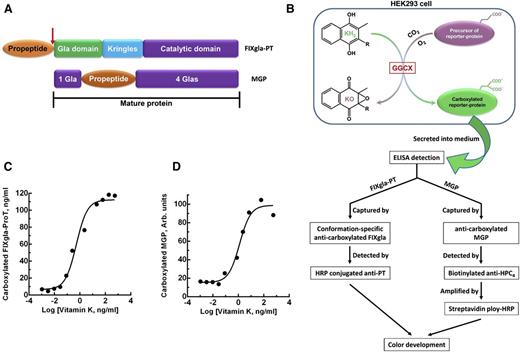
To study vitamin K–dependent carboxylation in the cellular milieu, we stably coexpressed 2 vitamin K–dependent proteins as reporter proteins in HEK293 cells (ATCC, CRL-1573, Manassas, VA). The first reporter protein is prothrombin (ProT), which is used to evaluate the carboxylation efficiency of coagulation factors. For detection purposes, we replaced the Gla domain of ProT with that of factor IX (FIXgla-ProT).21 This replacement allowed us to use a monoclonal antibody specific for the carboxylated Gla domain of FIX for quantitative detection. To increase reporter-protein stability, the protease cleavage sites at arginine residues 155, 271, and 320 of ProT were mutated to glutamine.22 The second reporter protein is MGP with a C-terminal HPC4 tag (EDQVDPRLIDGK). The complementary DNA of these reporter proteins were cloned into the mammalian dual-expression vector pVITRO1-MCS (InvivoGen, San Diego, CA) and were stably expressed in HEK293 cells. Hygromycin-resistant cell colonies were screened for high expression of both reporter proteins.
Generation of CRISPR-Cas9–mediated GGCX knockout reporter cell line
To study the function of GGCX and its naturally occurring mutants, we knocked out the endogenous GGCX gene in the HEK293 cells with dual reporter proteins (FIXgla-ProT/MGP-HEK293) using CRISPR-Cas9–mediated genome editing. Cas9 and the selected guide RNA (gRNA) sequence targeting GGCX in plasmid pX330-U6-Chimeric-BB-CBh-hSpCas9 (a gift from Feng Zhang, Addgene plasmid #42230)23 were transiently expressed in FIXgla-ProT/MGP-HEK293 cells. Forty-eight hours posttransfection, cells were treated with trypsin and filtered with a 30-µm filter to obtain single-cell suspensions. Diluted cell suspensions were plated into 96-well plates or seeded into 150-mm dishes for loss-of-function screening of GGCX knockout, as previously described.24 To verify GGCX knockout in the candidate cell colonies, genomic DNA was prepared using QuickExtract DNA extraction solution.25 The gRNA-targeting region in the genomic DNA was amplified by PCR, and the PCR products were cloned into TOPO cloning vector for sequence analysis. To determine the endogenous GGCX expression in the wild-type and GGCX knockout cells, cell lysates were loaded to sodium dodecyl sulfate-polyacrylamide gel electrophoresis for western blot analysis. Protein bands were probed by rabbit anti-GGCX polyclonal antibody (Proteintech Group Inc., Chicago, IL) and mouse anti-tubulin monoclonal antibody (GenScript Inc., Piscataway, NJ).
Cell-based GGCX activity assay
Carboxylation activity of GGCX or its variants was determined in the GGCX-deficient cell line as previously described,24 with minor changes. Briefly, plasmid DNA (pBudCE4.1-Met.Luc) carrying either wild-type or mutant GGCX was transiently transfected into the GGCX-deficient dual-reporter cells. Transfected cells were cultured with complete medium containing either 5 μg/mL vitamin K1 or with different concentrations of menaquinone, as indicated elsewhere. The cell culture medium was collected after 48 and 72 hours’ incubation and used directly for sandwich-based ELISA to quantitate carboxylated FIXgla-ProT and MGP, respectively. Wild-type GGCX activity was normalized to 100%.
To evaluate FIXgla-ProT carboxylation, we used, as the capture antibody, a conformation-specific monoclonal antibody that in the presence of calcium, recognizes only the fully carboxylated FIXgla domain (Green Mountain Antibodies, Burlington, VT). As the detecting antibody, we used horseradish peroxidase–conjugated sheep anti-human protein ProT (Affinity Biologicals Inc., Ancaster, Canada). As the capture antibody for measuring carboxylated MGP, we used a monoclonal antibody that specifically against carboxylated MGP sequence 35 through 49 (VitaK BV, Maastricht, the Netherlands). As the detecting antibody, we used biotinylated anti-HPC4 monoclonal antibody. Streptavidin poly-HRP (Pierce, Rockford, IL) was used to amplify the signal of MGP detection.
Fluorescence confocal microscopy and immunoblotting
To localize the expression of the wild-type GGCX and the M174R mutant (GGCXM174R), C-terminal tagged green fluorescent protein (GFP) fusion of these 2 proteins was transiently expressed in HEK293 cells on the cover slide. Forty-eight hours posttransfection, the cells were directly used for fluorescence confocal microscope imaging. Confocal microscopy was performed on a Zeiss LSM710 confocal laser-scanning microscope (Carl Zeiss Microimaging, Thornwood, NY). Images were collected using a 40×/1.2NA C-Apochromat objective lens. Visualization of the GFP was achieved by use of a 488-nm argon laser line for excitation, and the detector was set to collect emission at 493 to 530 nm.
To detect protein expression and stability, HPC4-tagged GGCX and GGCXM174 were transiently expressed in HEK293 cells. Forty-eight hours posttransfection, cells were washed twice in situ with phosphate-buffered saline (pH 6.8) and lysed with 1% Triton X-100 in the presence of a protease inhibitor on ice for 10 minutes. Cell lysate was directly used for sodium dodecyl sulfate-polyacrylamide gel electrophoresis and western blot detection. Protein bands were detected by anti-HPC4 mouse clonal antibody and visualized by horseradish peroxidase–conjugated goat anti-mouse antibody.
Results
Clinical summary
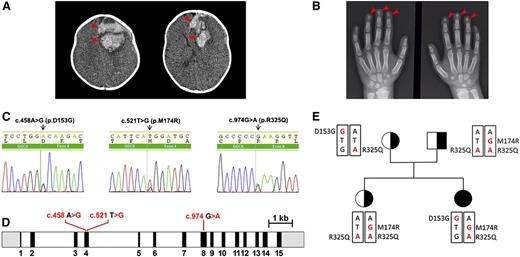
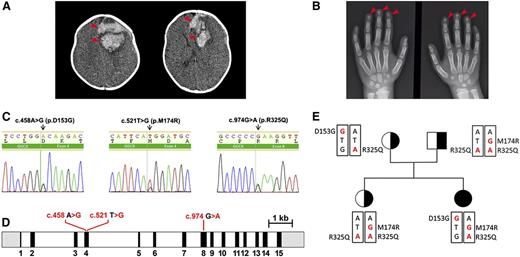
A 4-month-old girl, the first offspring of nonconsanguineous parents, presented pale and apathetic, and was hospitalized for vomiting and seizure. She showed no signs of malabsorption or hepatic disease. The patient’s hemoglobin concentration was 8.0 g/dL, her prothrombin activity was 11%, and her ratio of APTT was 1.65. A computed tomography scan of her brain showed a large area of bleeding (Figure 1A; see supplemental Figure 1A, available on the Blood Web site). Initially, she was treated with packed red blood cells and fresh frozen plasma, and underwent neurosurgery to drain the intracranial bleeding. A detailed survey of her clotting system showed VKCFD (Table 1). She was treated first with prothrombin complex concentrate and then was switched to oral vitamin K1. Her bleeding diathesis immediately improved, and she showed improvement in both clotting time and coagulation factor activity. Although no bleeding has occurred since the beginning of the vitamin K1 treatment, all coagulation factor activities reached up to 54% of the normal levels (Table 1). In addition to the coagulation diathesis, the patient also displayed characteristics of Keutel syndrome (Figure 1B; supplemental Figure 1B), which is usually attributed to a functional defect of MGP.14 Therefore, we measured the patient’s dp-ucMGP concentration in the circulation. To our surprise, this patient’s dp-ucMGP concentration is ∼14-fold higher than the average value in healthy children.26 Unlike her clotting factor deficiency, this dp-ucMGP excess cannot be corrected by supplementing with K vitamins (Table 1).
Radiologic images of clinical phenotypes, sequencing results of GGCX genotypes, and pedigrees of the patient. (A) Computed tomography brain scan at hospital admission, showing a large area of bleeding on the left frontal lobe (arrowheads) and deviation of the midline. (B) Hand x-ray showing short distal phalanges of the fingers (arrowheads), a characteristic of Keutel syndrome. (C) Chromatograms of the identified sequence variations demonstrating heterozygous mutations in GGCX at c.458 A>G, c.521 T>G, and C.974 G>A. (D) The intron-exon organization of GGCX with the location of the mutations as identified in the patient. The 5′ and 3′ untranslated regions are shown in grayscale, the exons are indicated by black rectangles, and the introns are represented by clear rectangles. (E) The pedigrees of the patient’s family. Squares, male; circles, females; black-filled circle, disease-affected patient; black half-filled square/circles, carrier. Mutated nucleotides are in red; mutated amino acid residues are indicated on the side.
Radiologic images of clinical phenotypes, sequencing results of GGCX genotypes, and pedigrees of the patient. (A) Computed tomography brain scan at hospital admission, showing a large area of bleeding on the left frontal lobe (arrowheads) and deviation of the midline. (B) Hand x-ray showing short distal phalanges of the fingers (arrowheads), a characteristic of Keutel syndrome. (C) Chromatograms of the identified sequence variations demonstrating heterozygous mutations in GGCX at c.458 A>G, c.521 T>G, and C.974 G>A. (D) The intron-exon organization of GGCX with the location of the mutations as identified in the patient. The 5′ and 3′ untranslated regions are shown in grayscale, the exons are indicated by black rectangles, and the introns are represented by clear rectangles. (E) The pedigrees of the patient’s family. Squares, male; circles, females; black-filled circle, disease-affected patient; black half-filled square/circles, carrier. Mutated nucleotides are in red; mutated amino acid residues are indicated on the side.
GGCX mutation identification
Genetic analysis of the GGCX gene in the patient and her family members detected 3 missense mutations (Figure 1C-E; supplemental Figure 2). Although both parents and the patient’s sister, born subsequently, were found to be asymptomatic, the patient and her mother had a heterozygous transition from A to G at position c.458 (p.D153G) in exon 4 of GGCX—a previously unreported mutation. Both siblings and their father displayed a heterozygous transversion from T to G at position c.521 (p.M174R), also in exon 4; this mutation had been previously reported as compound heterozygous mutation in a VKCFD patient.27 Additionally, a transition from G to A at position c.974 (p.R325Q) in exon 8 is homozygosis in the patient’s father and sister and is heterozygosis for the patient and her mother; this variant has no clinical relevance to bleeding disorders.28-30 Because Keutel syndrome is associated with mutations in MGP, we sequenced the MGP gene of our patient; no variation was found.
Establishment of the cell-based assay
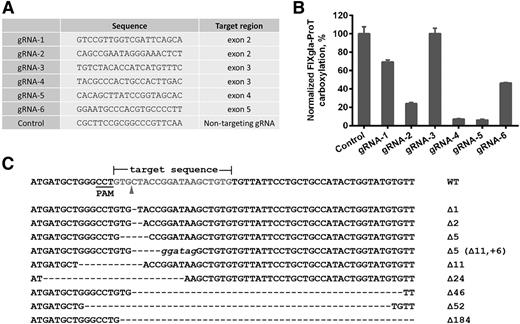
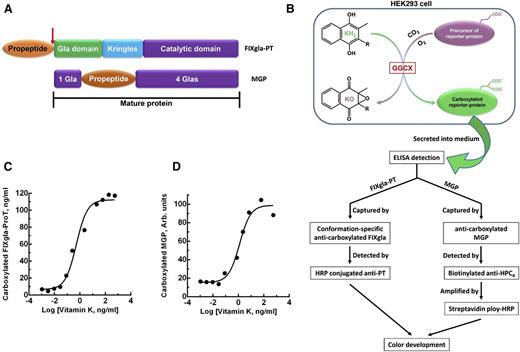
To better understand the correlations between GGCX genotypes and their clinical phenotypes, we established a cell-based assay to study GGCX’s function in its native milieu using natural protein substrates. We first stably coexpressed 2 structurally distinct vitamin K–dependent proteins as reporter proteins in HEK293 cells: a chimeric coagulation factor FIXgla-ProT and MGP (Figure 2A-B). Both reporter proteins are readily carboxylated when cells are supplemented with vitamin K1 (Figure 2C-D). We then knocked out the endogenous GGCX gene in this dual-reporter cell line using CRISPR-Cas9–mediated genome editing23 (supplemental Figure 3). To select an efficient targeting site for GGCX knockout, we examined the genome-editing efficacy of 6 optimized gRNA sequences31 (Figure 3A) by detecting the loss of reporter-protein carboxylation. Compared with the control, gRNA-4 and gRNA-5 knocked down more than 90% of carboxylation activity (Figure 3B), indicating effective GGCX targeting. We then used gRNA-5 to knock out the GGCX gene in our dual-reporter cells. Positive cell colonies of GGCX-deficient cells were obtained by loss-of-function screening and confirmed by genomic DNA sequencing (Figure 3C). The selected positive GGCX-deficient cell colonies express no detectable endogenous GGCX and are unable to carboxylate the reporter proteins (Figure 4). Expression of the exogenous GGCX in these GGCX-deficient cells restores carboxylation activity (Figure 4B), suggesting that other components of the vitamin K cycle in the cells as well as the reporter system, are intact.
Cell-based carboxylation activity assay with 2 reporter proteins. (A) Domain structure of the reporter proteins. Catalytic domain, region containing the serine protease catalytic triad; Gla domain, residues 1 through 46 of human factor IX containing 12 Gla residues; Kringles, regions of internal sequence homology; propeptide, residue −18 to −1 of human prothrombin. Arrow indicates the site that is proteolytically cleaved for protein maturation. MGP retains its propeptide within the mature protein and has Gla residues scattered around the propeptide. (B) Schematic diagram of reporter-protein carboxylation, secretion from HEK293 cells, and detected by sandwich-based ELISA. (C) FIXgla-ProT carboxylation in HEK293 cells in response to increasing concentrations of vitamin K1. (D) MGP carboxylation in HEK293 cells in response to increasing concentrations of vitamin K1.
Cell-based carboxylation activity assay with 2 reporter proteins. (A) Domain structure of the reporter proteins. Catalytic domain, region containing the serine protease catalytic triad; Gla domain, residues 1 through 46 of human factor IX containing 12 Gla residues; Kringles, regions of internal sequence homology; propeptide, residue −18 to −1 of human prothrombin. Arrow indicates the site that is proteolytically cleaved for protein maturation. MGP retains its propeptide within the mature protein and has Gla residues scattered around the propeptide. (B) Schematic diagram of reporter-protein carboxylation, secretion from HEK293 cells, and detected by sandwich-based ELISA. (C) FIXgla-ProT carboxylation in HEK293 cells in response to increasing concentrations of vitamin K1. (D) MGP carboxylation in HEK293 cells in response to increasing concentrations of vitamin K1.
CRISPR-Cas9–mediated GGCX knockout in HEK293 reporter cells. (A) Six optimized gRNA sequences for GGCX targeting and a nontargeting gRNA sequence as the control. (B) Carboxylation activity of HEK293 reporter cells targeted by different gRNAs, as shown in panel A. (C) Insertion and deletion mutations (indels) introduced by Cas9-gRNA-5 in the target site. The wild-type sequence is shown at the top. Dashes, deletions; italic lowercase letters, insertions. The sizes of indels are indicated to the right of each sequence.
CRISPR-Cas9–mediated GGCX knockout in HEK293 reporter cells. (A) Six optimized gRNA sequences for GGCX targeting and a nontargeting gRNA sequence as the control. (B) Carboxylation activity of HEK293 reporter cells targeted by different gRNAs, as shown in panel A. (C) Insertion and deletion mutations (indels) introduced by Cas9-gRNA-5 in the target site. The wild-type sequence is shown at the top. Dashes, deletions; italic lowercase letters, insertions. The sizes of indels are indicated to the right of each sequence.
Characterization of the GGCX knockout reporter cells. (A) Immunoblotting of wild-type (lane 1) and GGCX knockout (lane 2) HEK293 cells. Endogenous GGCX band is indicated by the arrow. (B) Carboxylation activity of GGCX-knockout cells (GGCX-KO) and the cells transfected with wild-type GGCX.
Characterization of the GGCX knockout reporter cells. (A) Immunoblotting of wild-type (lane 1) and GGCX knockout (lane 2) HEK293 cells. Endogenous GGCX band is indicated by the arrow. (B) Carboxylation activity of GGCX-knockout cells (GGCX-KO) and the cells transfected with wild-type GGCX.
Characterization of patient’s GGCX mutation
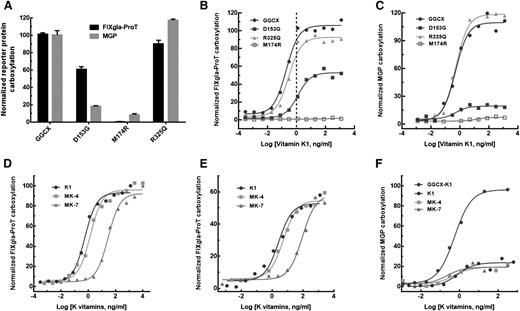
To evaluate how GGCX mutations impacted the clinical phenotypes of our patient, we transiently expressed each of the mutants in the GGCX-deficient cells and cultured these cells with 5 µg/mL vitamin K1. Compared with the wild-type enzyme, GGCXR325Q is almost fully active for the carboxylation of both reporter proteins (Figure 5A). This result suggests that the R325Q mutation does not contribute to either diathesis, which agrees with previous studies.28-30 In contrast, GGCXM174R carboxylates neither reporter protein in the presence of vitamin K1. This result suggests that the M174R mutation alone is unlikely to explain the patient’s clinical phenotypes because vitamin K1 supplementation improved her coagulation diathesis (Table 1). Interestingly, GGCXD153G has ∼60% activity for FIXgla-ProT carboxylation, but displays significantly decreased MGP carboxylation (∼15%); this result is consistent with the patient’s clinical result obtained from vitamin K1 administration, which offered up to 54% recovery of FIX activity but showed no improvement in MGP carboxylation (Table 1). These results suggest that when inherited alongside a null allele of GGCX (ie, M174R), it is the D153G mutation on the other allele that is the causative mutation for both VKCFD and Keutel syndrome in our patient.
Characterization of the 3 identified GGCX mutations using the established cell-based assay. (A) Carboxylation of FIXgla-ProT (black bars) and MGP (gray bars) by wild-type GGCX, GGCXD153G, GGCXM174R, and GGCXR325Q in GGCX-deficient cells in the presence of 5 µg/mL vitamin K1. (B) Carboxylation of FIXgla-ProT by wild-type GGCX (circles), GGCXD153G (solid squares), GGCXM174R (open squares), and GGCXR325Q (triangles) with increasing concentrations of vitamin K1; the vertical dashed line indicates the presumed physiological concentration of vitamin K1. (C) Carboxylation of MGP by the wild-type GGCX, GGCXD153G, GGCXM174R, and GGCXR325Q with increasing concentrations of vitamin K1 as in panel B. (D) Carboxylation of FIXgla-ProT by wild-type GGCX with increasing concentrations of vitamin K1 (circles), MK-4 (squares), and MK-7 (triangles). (E) Carboxylation of FIXgla-ProT by GGCXD153G with increasing concentrations of K vitamins as in panel D. (F) Carboxylation of MGP by GGCXD153G with increasing concentrations of K vitamins as in panel D. Carboxylation of MGP by the wild-type GGCX with vitamin K1 was used as a control (darker circles).
Characterization of the 3 identified GGCX mutations using the established cell-based assay. (A) Carboxylation of FIXgla-ProT (black bars) and MGP (gray bars) by wild-type GGCX, GGCXD153G, GGCXM174R, and GGCXR325Q in GGCX-deficient cells in the presence of 5 µg/mL vitamin K1. (B) Carboxylation of FIXgla-ProT by wild-type GGCX (circles), GGCXD153G (solid squares), GGCXM174R (open squares), and GGCXR325Q (triangles) with increasing concentrations of vitamin K1; the vertical dashed line indicates the presumed physiological concentration of vitamin K1. (C) Carboxylation of MGP by the wild-type GGCX, GGCXD153G, GGCXM174R, and GGCXR325Q with increasing concentrations of vitamin K1 as in panel B. (D) Carboxylation of FIXgla-ProT by wild-type GGCX with increasing concentrations of vitamin K1 (circles), MK-4 (squares), and MK-7 (triangles). (E) Carboxylation of FIXgla-ProT by GGCXD153G with increasing concentrations of K vitamins as in panel D. (F) Carboxylation of MGP by GGCXD153G with increasing concentrations of K vitamins as in panel D. Carboxylation of MGP by the wild-type GGCX with vitamin K1 was used as a control (darker circles).
To gain further insights on how the clinical phenotypes respond to vitamin K1 treatment, we titrated the identified GGCX mutants with different concentrations of vitamin K1. The response of GGCXR325Q to increasing vitamin K1 concentrations is similar to that of the wild-type enzyme for carboxylation of both reporter proteins (Figure 5B-C). In contrast, GGCXM174R cannot effectively carboxylate either of the reporter proteins. Compared with the wild-type enzyme, GGCXD153G requires ∼6-fold higher vitamin K1 concentrations to reach half-maximal carboxylation of FIXgla-ProT (half-maximal effective concentration, 1.26 ng/mL vs 0.198 ng/mL). At the presumed physiological concentration of vitamin K1 (∼1 ng/mL),28 both GGCXR325Q and the wild-type enzyme are fully functional for FIXgla-ProT carboxylation, whereas GGCXD153G only exhibits ∼20% activity (Figure 5B). Unfortunately, higher vitamin K1 concentrations failed to ameliorate MGP carboxylation for GGCXD153G (Figure 5C); this again agrees with our clinical results (Table 1).
Supplementation with menaquinone significantly increases the level of cMGP in the circulation of hemodialysis patients and healthy subjects.32,33 To test whether menaquinone could improve MGP carboxylation for our patient, we titrated GGCXD153G with different concentrations of menaquinone (MK-4 and MK-7) using our cell-based assay. Like the wild-type enzyme (Figure 5D), GGCXD153G (Figure 5E) can use MK-4 as efficiently as vitamin K1 for FIXgla-ProT carboxylation, but requires higher MK-7 concentrations to reach similar levels of carboxylation efficacy (half-maximal effective concentration, 2.61 ng/mL for vitamin K1 vs 89.0 ng/mL for MK-7). Unlike GGCX, none of these K vitamins improves MGP carboxylation for GGCXD153G (Figure 5F). These results again agree with the clinical results achieved by treating our patient with K vitamins (Table 1).
The M174R mutation disrupts GGCX’s structure
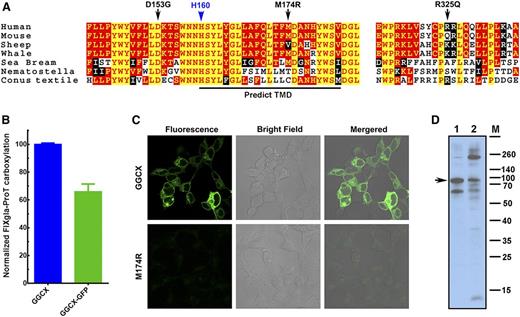
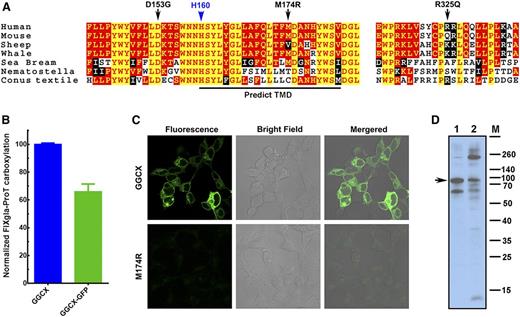
Our results suggest that the causative mutation for our patient’s VKCFD and Keutel syndrome is at residue D153, which is fully conserved in widely divergent species (Figure 6A). Although residue M174 is less conserved, GGCXM174R totally abolishes GGCX activity for the carboxylation of both reporter proteins. The M174R mutation introduces a charged residue in the predicted transmembrane helix (Figure 6A), which could disrupt the membrane topology of GGCX (supplemental Figure 4) and affect GGCX’s correct folding.34 To test this hypothesis, we fused GFP to the C-terminus of GGCX and GGCXM174R, expressed these fusion proteins in HEK293 cells, and examined protein localization and expression by fluorescence confocal microscopy and immunoblotting. Our cell-based assays show that the GGCX-GFP fusion retains more than 65% activity when compared with GGCX (Figure 6B). Although the wild-type GGCX is exclusively localized to the endoplasmic reticulum, the fluorescence signal of GGCXM174R diffuses over the entire cell with significantly less intensity (Figure 6C), suggesting the misfolding and degradation of the protein. Consistent with this interpretation, immunoblotting reveals that GGCXM174R shows more protein bands resulting from degradation and aggregation than the wild-type GGCX (Figure 6D), suggesting that the M174R mutation disrupts GGCX’s structure.
M174R mutation disrupts GGCX structure and folding. (A) GGCX conservation. Multiple-sequence alignment of GGCX near the identified mutations in 7 species. The amino acid sequences were aligned by CLUSTALW. Black arrows, GGCX mutations identified in the patient; blue arrow, the active site glutamate deprotonation residue H160; red with yellow background, completely conserved residues; yellow with red background, identical residues; white with black background, similar residues; black with white background, different residues. (B) Cell-based carboxylation activity of GGCX and GGCX-GFP fusion protein. (C) Fluorescence confocal microscopy image of GGCX-GFP (top) and GGCXM174R-GFP fusions (bottom). (D) Immunoblotting of GGCX (lane 1) and the GGCXM174R (lane 2). Full-length GGCX band is indicated by the arrow.
M174R mutation disrupts GGCX structure and folding. (A) GGCX conservation. Multiple-sequence alignment of GGCX near the identified mutations in 7 species. The amino acid sequences were aligned by CLUSTALW. Black arrows, GGCX mutations identified in the patient; blue arrow, the active site glutamate deprotonation residue H160; red with yellow background, completely conserved residues; yellow with red background, identical residues; white with black background, similar residues; black with white background, different residues. (B) Cell-based carboxylation activity of GGCX and GGCX-GFP fusion protein. (C) Fluorescence confocal microscopy image of GGCX-GFP (top) and GGCXM174R-GFP fusions (bottom). (D) Immunoblotting of GGCX (lane 1) and the GGCXM174R (lane 2). Full-length GGCX band is indicated by the arrow.
Discussion
GGCX is a 758-amino acid integral membrane protein that spans the endoplasmic reticulum membrane 5 times.35 Since the purification of the enzyme and cloning of the gene in 1991,36,37 significant progress has been made in understanding how GGCX interacts with its substrates and achieves catalysis.17 Most of our knowledge concerning GGCX’s function, however, has been obtained from an in vitro activity assay using the pentapeptide FLEEL as its substrate. Thus, our understanding of the carboxylation of vitamin K–dependent proteins in their native milieu is lacking. For example, GGCX recognizes its substrate through a relatively tight binding to the propeptide of the vitamin K–dependent proteins, which tethers the substrate to the enzyme.38 Although the apparent affinities of the propeptides of vitamin K–dependent proteins for GGCX vary more than 100-fold in vitro,39,40 these proteins appear to be fully carboxylated under physiological conditions in vivo. In addition, it is not clear why some naturally occurring GGCX mutations cause deficiencies of the carboxylation of coagulation factors (resulting in bleeding disorders), whereas others cause undercarboxylation of extrahepatic vitamin K–dependent proteins such as MGP (resulting in vascular calcification and PXE-like syndrome). Our goal in this study was to develop an in vivo activity assay for studying the function of GGCX and to clarify the clinical consequences of the naturally occurring GGCX mutations in a cellular environment.
In our cell-based assay, we selected 2 structurally distinct vitamin K–dependent proteins, FIXgla-ProT and MGP, as reporter proteins. FIXgla-ProT is carboxylated in the liver and has all its Gla residues concentrated in the N-terminal Gla domain preceded by the propeptide, which will be cleaved in the mature protein after carboxylation. MGP, which is carboxylated in extrahepatic tissues, retains its propeptide within the mature protein and has its Gla residues scattered on both sides of the propeptide (Figure 2A). We expect that these 2 reporter proteins may be carboxylated differently by GGCX, and that certain mutations in GGCX could have different effects on their carboxylation. Our results show that at optimal vitamin K concentrations, GGCXD153G has ∼60% activity for FIXgla-ProT carboxylation, but only exhibits ∼15% activity for MGP carboxylation (Figure 5). This suggests that the D153G mutation might affect the reporter protein’s propeptide binding and/or glutamate carboxylation. Because the propeptide-binding region of GGCX is located at the C-terminus between residues 495 and 513,41 it is unlikely that the mutation at residue 153 of the N-terminus affects propeptide binding. It has been proposed that the highly conserved residue H160 (Figure 6A) is the catalytic residue for glutamate deprotonation42 ; thus, it is possible that a mutation in the nearby residue (D153G) could affect glutamate deprotonation and carboxylation. Because our reporter proteins have distinct glutamate distributions within their molecules, the D153G mutation could affect the protein’s carboxylation differently, as we observed in both our cell-based assay (Figure 5) and in our patient (Table 1). Another possibility that 1 GGCX mutation causes different effects on the carboxylation of 2 reporter proteins could be attributed to the detailed carboxylation kinetic mechanisms of multiple glutamates in these reporter proteins during 1 substrate binding event. Overall, these results suggest that our reporter proteins can successfully distinguish the functional defects of GGCX mutations.
To evaluate the established cell-based assay for studying the clinical consequences of GGCX mutations, we compared the results from our cell-based assay with the clinical results of our patient. At the physiological vitamin K1 concentration, GGCX-deficient cells expressing the causative mutant GGCXD153G exhibit ∼20% activity for coagulation factor carboxylation; higher vitamin K1 concentrations recover the activity up to ∼60% (Figure 5). This is comparable to the patient’s clinical results: the baseline activity of FIX was 12%, and it increased to 53% after supplementation with high-dose vitamin K1 (Table 1). Additionally, our cell-based assay shows that higher concentrations of K vitamins exhibit only a moderate effect on MGP carboxylation (Figure 5C,F). This again agrees with our clinical results: that high-dose K vitamins failed to increase the carboxylation of MGP (Table 1). These results together suggest that our cell-based assay is a good system for the functional study of GGCX in vivo.
It is worth noting that vitamin K1 supplementation failed to improve the carboxylation of anticoagulant proteins (C and S) in our patients (Table 1). This cannot be simply explained by the differences of the propeptide affinities of vitamin K–dependent proteins to GGCX that were obtained by in vitro activity assay. Protein C and ProT, as well as protein S and factor VII, have similar propeptide affinities to GGCX39 ; both ProT and factor VII show substantial improvement of carboxylation after high-dose vitamin K1 administration (Table 1). In addition, replacing the propeptide of factor X with the more weakly binding ProT propeptide improved the carboxylation efficiency of factor X from 30% to 85% in HEK293 cells.43 However, a similar strategy failed to increase the carboxylation efficiency of FIX.44 Therefore, the characteristics of propeptide binding to GGCX may differ in vitro and in vivo. Furthermore, glutamate binding45 and a potential propeptide-independent binding of vitamin K–dependent proteins to GGCX46 also contribute to the efficiency of vitamin K–dependent carboxylation in vivo. With our cell-based assay, we expect to explore this complicated event by mutating residues at the identified corresponding region in GGCX and examining their effect on reporter protein carboxylation.
The most abundant form of K vitamin in the liver is vitamin K1.47 Vitamin K1 is the main supplement used to treat VKCFD patients to improve coagulation factor carboxylation. However, menaquinones are believed to be important for vitamin K–dependent carboxylation in extrahepatic tissues.48 For example, it has been observed that administration of menaquinone, but not vitamin K1, improves MGP carboxylation and reduces both cardiovascular mortality and coronary calcification.32,49,50 However, results from our cell-based assay show that none of the K vitamins tested improve MGP carboxylation for GGCXD153G (Figure 5F). This observation is similar to the response of our patient to vitamin K treatment (Table 1). In addition, it has been shown that supplementation of high-dose vitamin K did not increase the level of cMGP in patients with Keutel syndrome.51 Therefore, the improvement of MGP carboxylation by menaquinones may account for extrahepatic cell utilization of these K vitamins52 for wild-type GGCX carboxylation. When the defects are caused by mutations in GGCX or MGP, menaquinones cannot ameliorate the carboxylation of MGP.
In summary, we identified and characterized GGCX mutations in a patient with both bleeding and nonbleeding disorders using a GGCX-deficient cell-based assay with dual reporter proteins. Our results indicated that 1 GGCX mutation (D153G) causes 2 distinct clinical phenotypes and explained the effectiveness of clinical treatments for our patient with vitamin K. The established cell-based assay provides a useful tool for studying the correlation between GGCX genotypes and their clinical phenotypes in vivo. Studying the functional consequences of GGCX mutations using this assay will allow us to increase our knowledge about these mutations’ clinical phenotypes in a way not previously possible, and could also offer insights into treating patients carrying different GGCX mutations.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the patient and her family for their participation. We also thank Dr Brian Ingram and Dr Guomin Shen for helpful discussions, Elbio A. D’Amico and Tania R. F. Rocha of the Laboratório de Hemostasia e Trombose do Serviço de Hematologia do Hospital das Clínicas for their help regarding clotting-factor assays, and Dr Heath Sledge for editing the manuscript.
This work was supported by a grant from the National Institutes of Health, National Heart, Lung, and Blood Institute (grant HL077740) (D.W.S. and J.K.T.).
Authorship
Contribution: J.-K.T. designed the study, analyzed the data, and wrote the manuscript; J.D.A.C. provided clinical information and performed coagulation tests; D.-Y.J. and J.-K.T. generated the GGCX knockout cell line and performed all the cell-based assays and GGCX mutant characterizations; C.D.M. generated and analyzed DNA sequencing data; C.V. determined and analyzed the dp-ucMGP of the patient; and D.W.S. oversaw the entire project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jian-Ke Tie, Department of Biology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599; e-mail: jktie@email.unc.edu.
References
Author notes
J.-K.T. and J.D.A.C. contributed equally to this work.