Abstract
Recent advances in genetic engineering have enabled the delivery of clinical trials using patient T cells redirected to recognize tumor-associated antigens. The most dramatic results have been seen with T cells engineered to express a chimeric antigen receptor (CAR) specific for CD19, a differentiation antigen expressed in B cells and B lineage malignancies. We propose that antigen expression in nonmalignant cells may contribute to the efficacy of T-cell therapy by maintaining effector function and promoting memory. Although CAR recognition is limited to cell surface structures, T-cell receptors (TCRs) can recognize intracellular proteins. This not only expands the range of tumor-associated self-antigens that are amenable for T-cell therapy, but also allows TCR targeting of the cancer mutagenome. We will highlight biological bottlenecks that potentially limit mutation-specific T-cell therapy and may require high-avidity TCRs that are capable of activating effector function when the concentrations of mutant peptides are low. Unexpectedly, modified TCRs with artificially high affinities function poorly in response to low concentration of cognate peptide but pose an increased safety risk as they may respond optimally to cross-reactive peptides. Recent gene-editing tools, such as transcription activator–like effector nucleases and clustered regularly interspaced short palindromic repeats, provide a platform to delete endogenous TCR and HLA genes, which removes alloreactivity and decreases immunogenicity of third-party T cells. This represents an important step toward generic off-the-shelf T-cell products that may be used in the future for the treatment of large numbers of patients.
Engineering of T-cell specificity
Rapid improvements of gene transfer technologies have provided a robust platform to redirect the specificity of primary T cells.1 This has overcome a major obstacle for targeted T-cell therapy, posed by the relatively low frequency of cancer-reactive T cells that are naturally present in patients or that can be induced by vaccination. Retro- and lentiviral gene transfer platforms have been developed to achieve the expression of cancer-reactive T-cell receptors (TCRs) and chimeric antigen receptors (CARs) in primary T cells, generating therapeutic cellular products with a high level of tumor specificity.2 There is now the opportunity to direct the therapeutic power of T-cell therapy toward defined cancer antigens and thus avoid the toxicity of donor lymphocyte infusion, which is caused by the alloreactivity of the polyclonal TCR repertoire of infused T cells.
TCRs or CARs to target malignant cells
TCRs recognize peptide fragments presented by HLA molecules.3 An evolutionary advantage of this mode of antigen recognition enables T cells to recognize and attack virus-infected cells, even when viral proteins are “hidden” inside cells and absent from the surface.4 Similarly, this mode of recognition renders intracellular cancer proteins susceptible for targeted attack by TCRs (Figure 1). For example, most cancer testis antigens are not expressed on the surface of tumor cells but are nevertheless efficiently targeted by TCRs, but not by CARs.5 Similarly, intracellular proteins such as Wilms tumor antigen-1 and minor histocompatibility antigens have been validated in preclinical experiments as attractive targets for the treatment of hematologic malignancies.6-9 TCR gene therapy trials targeting these antigens are currently open for recruitment of leukemia patients.
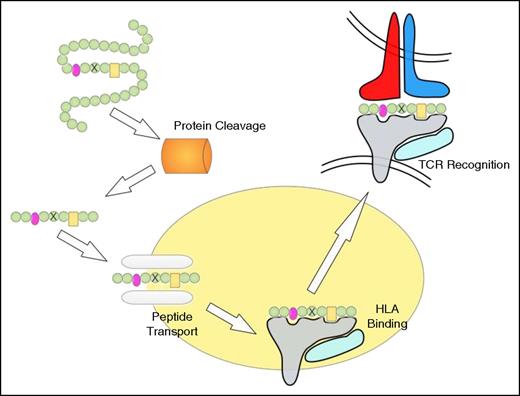
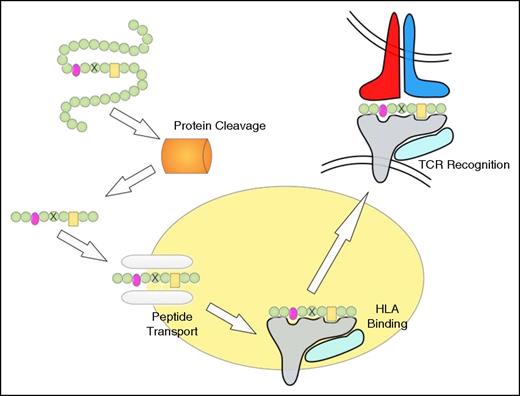
Peptide processing, HLA binding, and TCR recognition. The proteasome degrades proteins to produce peptide fragments, which are transported from the cytosol into the endoplasmic reticulum (ER) by the transporter associated with antigen processing (TAP) complex. Inside the ER, peptides bind to HLA class I molecules, which are then transported to the cell surface where the HLA/peptide structure is recognized by TCRs. The peptide residues important for HLA binding are indicated in pink and yellow, and the mutated residue is indicated by a cross. Note that the proteasome may not cleave at the appropriate position and that the mutation-containing peptide may not be transported by TAP or fail to bind to HLA.
Peptide processing, HLA binding, and TCR recognition. The proteasome degrades proteins to produce peptide fragments, which are transported from the cytosol into the endoplasmic reticulum (ER) by the transporter associated with antigen processing (TAP) complex. Inside the ER, peptides bind to HLA class I molecules, which are then transported to the cell surface where the HLA/peptide structure is recognized by TCRs. The peptide residues important for HLA binding are indicated in pink and yellow, and the mutated residue is indicated by a cross. Note that the proteasome may not cleave at the appropriate position and that the mutation-containing peptide may not be transported by TAP or fail to bind to HLA.
The TCR recognition is focused on short linear peptide epitopes presented by HLA class I molecules (9-10 amino acid peptides) and HLA class II molecules (15-18 amino acids).10,11 Although some of these peptide residues mediate HLA binding, other residues interact primarily with the complementary determining region 3 of the TCR, providing appropriate engagement required for T-cell activation.12 Both HLA binding and TCR interaction can be exquisitely sensitive to single amino acid substitutions, which is an important consideration for cancer immunology. Although patient T cells are tolerant to self-peptides derived from self-proteins, point mutations in tumor cells resulting in single amino acid changes can elicit robust T-cell responses.13-20 There are 2 mechanisms whereby point mutations can generate immunogenic epitopes to which patient T cells are not tolerant. First, mutations may generate novel TCR contact residues and thus produce immunogenic neoepitopes, or, alternatively, they may create novel HLA-binding residues resulting in the presentation of peptides in tumor cells that are absent in normal tissues. Because of recognition of linear peptide sequences, TCRs can potentially target the mutational landscape associated with cancer development, although, as discussed subsequently, cellular mechanisms of peptide production and transport impose considerable limitations. In contrast to TCRs, point mutations largely escape antibody recognition and thus CAR targeting because the vast majority of mutated proteins are intracellular and because antibodies are less effective in the specific recognition of point mutations in otherwise unaltered self-proteins.
Although the HLA-dependent antigen recognition gives TCRs access to target antigens that escape CAR recognition, it is also a major disadvantage because therapeutic TCR can only be used in a limited number of patients with the appropriate HLA alleles. To date, the vast majority of TCRs that are in clinical trials or in preclinical testing are restricted by HLA-A*0201, which is found in ∼45% of white people. Therapeutic TCRs restricted by 4 common HLA class I alleles (A*0201, A*0301, A*2402, and B*0702) would substantially extend the applicability of TCR gene therapy and cover >90% of the white population in the United States.21
CARs recognize their antigen directly and can be used for the treatment of all malignancies bearing the relevant antigen irrespective of patients’ HLA genetics. CARs have the further advantage that they do not mispair with endogenous TCR chains, a mechanism that can create novel specificities in TCR gene-modified primary T cells. Although clinical trials have not yet demonstrated toxicity because of TCR mispairing, it is clear from murine model experiments that it can cause severe graft-versus-host disease (GVHD)–like pathology.22
Clinical experience of TCR gene therapy
Overviews of the ongoing TCR gene therapy trials in hematologic malignancies and in solid tumors have been published recently.23-25 The first TCR gene therapy trial was performed in melanoma, using a TCR specific for the melanocyte-specific differentiation antigen MART-1.26 The results of all published trials to date showed that TCR gene-modified patient T cells can have clinical activity and reduce tumor burden. However, it is clear that therapeutic TCRs have not yet delivered the dramatic and lasting anticancer responses that have been seen with CARs targeting CD19 on chronic lymphocytic leukemia (CLL), acute lymphoblastic leukemia (ALL), and B-cell lymphoma.27-30 Clinical trials of NY-ESO-1 TCR gene therapy in melanoma and synovial cell carcinoma, and in multiple myeloma patients undergoing autologous stem cell transplantation, have shown clear antitumor activity.31,32 In the myeloma study, disease progression was correlated with loss of persistence of the gene-modified T cells or with loss of NY-ESO-1 expression in the myeloma cells, suggesting that immune editing allowed myeloma cells to escape T-cell attack. It is important to note that all completed and ongoing TCR gene therapy trials target tumor-associated antigens that are also expressed to various extents in normal tissues. The physiological expression of TCR-targeted antigens poses the risk of “on-target” immune pathology as has been observed with TCR-targeting of MART1, glycoprotein 100, and carcinoembryonic antigen.33-35 However, we need to consider that antigen-expression in normal tissues can also enhance the efficacy of adoptive therapy with engineered T cells.
Self-antigen expression in normal tissues
To date, the highest level of clinical efficacy of any form of immunotherapy has been achieved with T cells engineered with CARs specific for CD19. In this case, control of B-cell malignancies is associated with the elimination of CD19-positive normal B cells and persistence of transferred T cells.30,36 It is not known from the human studies whether the continued elimination of B cells might trigger the sustained T-cell persistence. We would like to speculate that the interaction with normal B cells, which can provide T-cell costimulation and function as efficient antigen-presenting cells,37 might contribute to promoting the expansion and maintenance of the therapeutic T cells in vivo (Figure 2). Similar to CD19-targeted immunotherapy, successful immunotherapy of melanoma is associated with vitiligo caused by the T-cell recognition of tissue-specific antigens in normal melanocytes.38,39 Interestingly, studies in murine models have indeed provided evidence that the interaction with melanocytes provided T-cell stimulation and contributed to sustaining the memory potential of the therapeutic T cells.40
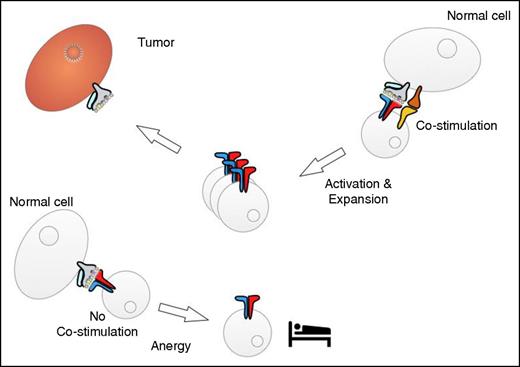
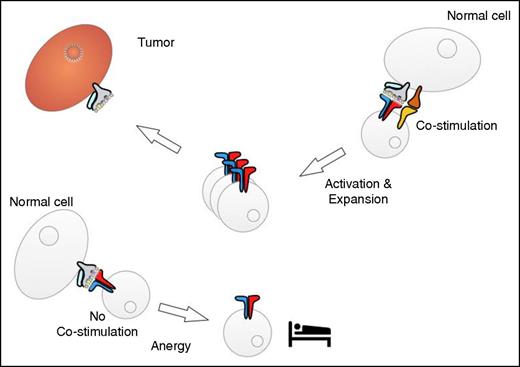
Interaction of tumor-reactive T cells with normal tissues. Cells in normal tissues may be able to provide costimulation, which results in T-cell activation and expansion (right). The interaction with cells that do not provide costimulation might induce a state of “sleepy” T-cell anergy (left).
Interaction of tumor-reactive T cells with normal tissues. Cells in normal tissues may be able to provide costimulation, which results in T-cell activation and expansion (right). The interaction with cells that do not provide costimulation might induce a state of “sleepy” T-cell anergy (left).
However, the expression of self-antigens in tissues and cell types that are unable to provide T-cell costimulation may result in the induction of anergy (Figure 2). Again, this was demonstrated in a murine model where adoptive transfer of TCR-engineered T cells specific for the MDM2 antigen resulted in the loss of T-cell function.41 It was shown that loss of T-cell function was driven by MDM2 antigen expression in nonhematopoietic tissues, whereas hematopoietic cells promoted T-cell function. The lack of costimulation and potential induction of anergy may be particularly relevant for T cells engineered with TCRs, whereas the engineering with CARs that contain costimulatory signaling domains may protect T cells from anergy induction.
For TCR gene therapy, it is therefore advisable to consider the expression pattern of therapeutic target antigens in the context of possible toxicity and to also take into account the impact on the modulation of T-cell function. We suggest that it may be desirable for tumor-associated self-antigens to be expressed in normal cells that are capable of T-cell stimulation and whose loss, as a consequence of T-cell attack, does not cause untreatable toxicity. The observation that antigen expression in the hematopoietic compartment can promote T-cell function suggests that lineage-specific antigens may be good targets for the treatment of hematologic malignancies. The haploidentical transplantation protocol that was recently used for adoptive transfer of nonengineered T cells could potentially provide an excellent platform to achieve the selective TCR targeting of leukemia.42 For example, it would be possible to engineer donor T cells with a TCR specific for a hematopoietic-specific antigen presented by an HLA class I allele that is expressed by the patient but not by the donor. In this case, the engineered T cells would selectively attack patient leukemia and hematopoietic cells but spare donor cells that lack the HLA molecule required for the recognition of the hematopoietic antigen.
Achieving unique tumor specificity
Chronic myeloid leukemia (CML) is characterized by the breakpoint cluster region (BCR)/Abelson (ABL) translocations that often produce the same mutant protein in patients.43 The targeting of this mutant protein with small-molecule inhibitors such as imatinib was a first example of a highly disease-specific therapy resulting in CML control in the majority of patients. The BCR/ABL fusion protein also contains linear peptide sequences that are unique to CML and absent in all nonmalignant cells. Despite substantial efforts to exploit fusion peptides for vaccination and to identify fusion-specific T-cell responses, the clinical results have been disappointing, and clear evidence of fusion-specific T cells capable of recognizing and killing CML remains absent.44 This is puzzling, considering that mutation-specific responses by both CD4+ helper T cells and CD8+ cytotoxic T cells have now been clearly identified in patients with melanoma and gastrointestinal cancer.13,16,19,20 Why is it, therefore, that BCR/ABL “escapes” T-cell immunity when other mutations trigger robust T-cell responses?
There are a number of requirements that a peptide has to meet to function as an epitope for T-cell attack (Figure 1). The proteasome has to cleave the protein to generate the appropriate peptide, which then has to interact productively with the TAP complex for transport into the ER, and inside the ER, peptides have to display sufficient binding affinity for HLA molecules to compete successfully with the large number of normal self-peptides generated from the set of 10 000 to 15 000 proteins that are expressed in human cells.45 The following considerations illustrate that the majority of cancer mutations are predicted to be invisible to immune recognition. Although tumors such as lung cancer and melanoma harbor ∼30 000 somatic mutations, the protein-encoding exome represents 1% of the entire genome, indicating that ∼300 mutations occur in protein-coding sequences. Of the 300 DNA alterations, only the nonsynonymous mutations and frameshifts will change amino acids and create an altered protein. Proteasome-mediated cleavage of the protein needs to release the mutation-containing peptides with the correct C-terminal residues required for HLA binding (Figure 1).46 This, however, is often not the case, and it has been demonstrated that proteasome cleavage can instead destroy TCR-recognized peptide epitopes.47,48 The efficiency of peptide transport from the cytosol into the ER is the next rate-limiting step, and experiments have demonstrated that certain amino acids in peptides can greatly decrease transport efficacy.49 Finally, HLA binding typically requires preferred peptide residues to function as anchors that mediate HLA binding; usually only 2 of the 20 possible amino acids can mediate efficient anchor function.50 Together, these considerations provide some explanation of why BCR/ABL fusion peptides may fail efficient HLA presentation. It also explains published studies of the immunogenicity of the cancer mutagenome, which have shown that only 0.3% to 1.3% of mutated sequences induced CD8 T-cell responses and only 0.5% of mutated peptides induced detectable responses by CD4 T cells.13,16,19,20
The frequency of mutations in hematologic malignancies is 10 to 20 times lower than melanoma and lung cancer. Multiple myeloma has ∼3000 mutations in the genome, whereas the frequency in acute myeloid leukemia, ALL, and CLL is ∼1500 to 2000 mutations.51 Despite this reduced mutation load, it is premature to conclude that neopeptides cannot function as targets for TCR gene therapy in hematologic malignancies. It is possible that the mutation-specific T cells detected in melanoma patients are directed against immune-dominant peptides that are presented at high levels. This would be similar to T-cell responses in viral infections that are often directed against dominant viral peptides. In this case, subdominant T-cell epitopes are detected after immunization with vaccines lacking the immune-dominant peptides.52 It is therefore possible that mutation-containing neopeptides that are inefficiently presented may serve as targets for TCR gene therapy, even when they do not stimulate T-cell responses in patients. The targeting of these subdominant neoepitopes would require high-avidity TCRs that can activate T-cell effector function at low peptide concentration. The immunization of mice transgenic for HLA and the human TCR gene repertoire, or the in vitro immunization of T cells from healthy donors, may provide a source for such high-avidity TCRs.5,53
TCRs to recognize low antigen concentrations
The affinity and the expression level of therapeutic TCRs are 2 key parameters that determine how much antigen is needed for the triggering T-cell effector function. Several engineering strategies have been employed to enhance the level of TCR expression on the T-cell surface. This includes codon optimization, introduction of an additional disulfide bond between the TCR chains, and the introduction of murine residues into the constant region domain.54-57 Despite these steps, there are still remarkable differences in in the expression levels achievable with TCRs that differ only in the variable region domains.58 We have performed extensive comparisons between poorly and strongly expressed human TCRs and have been able to identify key residues affecting the level of surface expression. Interestingly, these residues are outside the complementary determining regions of the variable domains and are therefore accessible to replacements without affecting T-cell specificity. Thus, the replacement of key residues in the framework of the variable region can improve TCR expression and enhance antigen-specific effector function.
An alternative strategy to enhance TCR expression is the provision of additional CD3 molecules in engineered T cells. We have shown that the transfer of TCR and CD3 genes into primary T cells enhanced TCR expression and improved recognition of low concentrations of antigen, which resulted in augmented tumor protection in vivo.59 However, additional CD3 also leads to enhanced expression of endogenous and mispaired TCR, thus increasing the risk of potential toxicity. The most effective strategy to prevent this risk is the disruption of endogenous TCR genes using zinc finger nucleases, transcription activator–like effector nucleases (TALENs), or clustered regularly interspaced short palindromic repeats (CRISPR)–Cas9 technologies.60
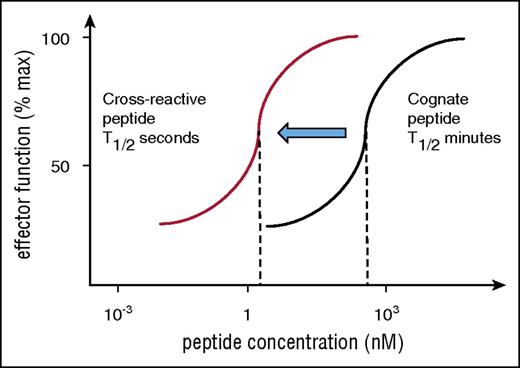
Altering TCR affinity is another strategy that has been used to achieve T-cell stimulation by low antigen concentration. Unexpectedly, experiments have shown that high affinities that increase the TCR binding to HLA peptide from the “physiological” half-life of seconds into the range of minutes and hours result in impaired ability to trigger T-cell stimulation when the peptide concentration is low.61 As a consequence, T cells expressing modified TCRs with affinities similar to that of antibodies are efficiently stimulated by high doses of cognate peptide but fail to respond to low-dose peptide (Figure 3). At a low dose of cognate peptide, 1 HLA/peptide complex needs to stimulate multiple TCR by a mechanism of serial triggering, which is disrupted when the half-life of TCR binding to HLA peptide is too long.62 Therefore, artificially high affinity not only impairs response to low concentration of cognate peptide, but it also enhances the risk of optimal triggering by cross-reactive peptides (Figure 3). Compared with the binding of cognate antigen, cross-reactivity usually occurs at lower affinity and shorter half-life and may therefore fall into the optimal range for serial triggering required for activation at low peptide concentration. In other words, artificially affinity-matured TCR may be more effective in stimulating T-cell responses against cross-reactive peptides compared with cognate peptides. This might have contributed to the fatal toxicities that have been seen with an affinity-matured MAGE-3A TCR that cross-reacted against MAGE-A12 in the brain and against Titin expressed in heart tissue.63,64
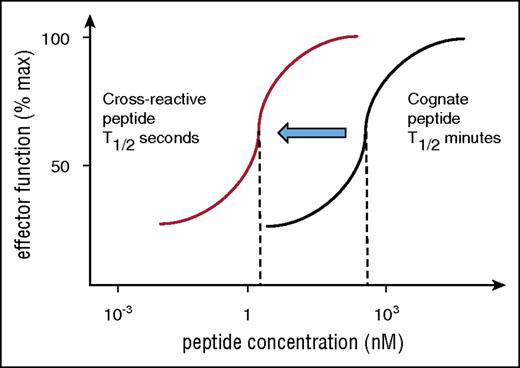
Risk of cross-reactivity by affinity-matured TCR. Engineered TCRs with artificially high affinity require high peptide concentration to stimulate T-cell responses (black titration curve indicates stimulation with cognate peptide). This is because of the long half-life of binding, which prevents sequential engagement of several TCRs with a single HLA/peptide ligand. Cross-reactive peptides are expected bind the same TCR with reduced affinity and binding half-life and may fall into the optimal range for sequential TCR engagement that is required for T-cell activation at low peptide concentration. Hence, the red titration curve indicates that the affinity-matured TCR is triggered by a lower concentration of cross-reactive peptide compared with cognate peptide (black curve).
Risk of cross-reactivity by affinity-matured TCR. Engineered TCRs with artificially high affinity require high peptide concentration to stimulate T-cell responses (black titration curve indicates stimulation with cognate peptide). This is because of the long half-life of binding, which prevents sequential engagement of several TCRs with a single HLA/peptide ligand. Cross-reactive peptides are expected bind the same TCR with reduced affinity and binding half-life and may fall into the optimal range for sequential TCR engagement that is required for T-cell activation at low peptide concentration. Hence, the red titration curve indicates that the affinity-matured TCR is triggered by a lower concentration of cross-reactive peptide compared with cognate peptide (black curve).
TCR-transduced CD4+ and CD8+ T cells
There is increasing evidence for a key role of CD4+ T cells in cancer immunity. Data from melanoma trials have suggested that adoptive transfer of both CD4+ and CD8+ T-cell subsets provided efficient antitumor activity. More recently, the adoptive transfer of CD4+ T cells specific for a mutation present in cholangiocarcinoma resulted in impressive clinical responses.18 A side-by-side comparison of adoptive therapy with antigen-specific CD4+ or CD8+ T-cell subsets in a murine melanoma model showed that the former were more effective in eradicating established tumors.65 The separation of the function of CD4+ and CD8+ T-cell subsets into production of helper cytokines and delivery of cytotoxicity is no longer valid, as data in humans and mice have demonstrated efficient cytotoxicity mediated by CD4+ T cells.66
These observations have provided a rationale for clinical trials of adoptive therapy with CD4+ T cells after allogeneic stem cell transplantation, and the results of these trials have shown that donor CD4+ T cells can mediate antileukemic effects with reduced risk of GVHD. However, the induction of HLA class II expression in nonhematopoietic tissues caused by cytomegalovirus reactivation was associated with GVHD.67
TCR engineering offers the opportunity to transfer HLA class I–restricted TCRs into CD4+ T cells, thus redirecting their specificity against the same target epitopes that are normally recognized by CD8+ T cells.68,69 Consequently, activation of CD4+ T-cell function is no longer dependent on antigen-presentation by HLA class II, which is frequently absent in tumor cells. A possible disadvantage of this strategy is that HLA class I is widely expressed in most tissues, thus extending possible toxicities of therapy with redirected CD4+ T cells or impairing their functional potential as a consequence of interaction with cells and tissues that can induce T-cell anergy (Figure 2).
Genome editing to modulate T-cell function
The first successful gene deletion in primary T cells was achieved using zinc finger nucleases to disrupt the endogenous TCR in engineered T cells expressing a therapeutic TCR.60 The more recently developed TALEN and CRISPR technologies are likely to increase the efficiency of gene editing in T cells. A recent study has used the TALEN platform to disrupt the expression of endogenous TCR and of CD52, which is recognized by alemtuzumab antibodies that are used to suppress immune rejection after allogeneic stem cell transplantation.70 Disruption of the endogenous TCR expression removed the ability of allogeneic T cells to cause GVHD, and the lack of CD52 made them resistant to alemtuzumab-mediated immune suppression. The TALEN-engineered third-party T cells were equipped with a CD19-specific CAR, enabling them to attack CD19-expressing ALL cells in an infant who had relapsed after allogeneic stem cell transplantation. This first example of combining lentiviral gene transfer with TALEN-mediated gene disruption demonstrates the feasibility of employing genetic engineering to add and remove genes with the goal to maximize the functional profile of therapeutic T cells (Figure 4). The design of third-party T cells as “off the shelf” therapeutics will require the complete removal of endogenous TCR to avoid GVHD pathology and also the removal of HLA class I and class II antigens to reduce immunogenicity and thus reduce the risk of rejection. The introduction of ligands for killer cell inhibitory receptors, such as nonclassical HLA-G, might reduce the risk of natural killer cell–mediated rejection triggered by the absence of classical HLA molecules.
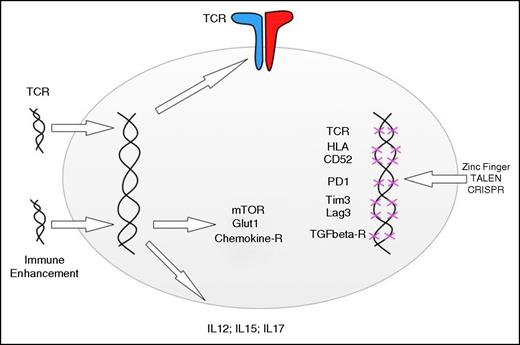
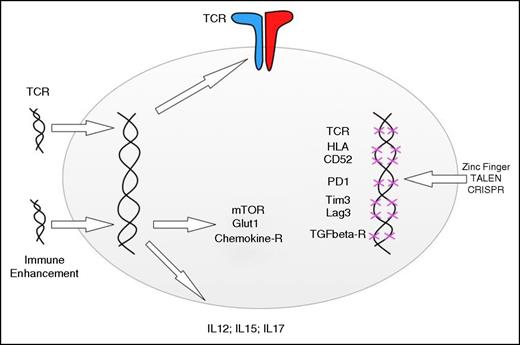
Adding and deleting genes to enhance T-cell function. Retro- and lentiviral gene transfer can be used to redirect the specificity of T cells and the metabolic functions, response to chemokines, and cytokine secretion. Gene disruption technologies can be used to delete endogenous TCR genes, HLA genes, and genes involved in negative T-cell regulation. The indicated genes are simply examples, and the editing technologies can be applied to add or disrupt any gene involved in T-cell function.
Adding and deleting genes to enhance T-cell function. Retro- and lentiviral gene transfer can be used to redirect the specificity of T cells and the metabolic functions, response to chemokines, and cytokine secretion. Gene disruption technologies can be used to delete endogenous TCR genes, HLA genes, and genes involved in negative T-cell regulation. The indicated genes are simply examples, and the editing technologies can be applied to add or disrupt any gene involved in T-cell function.
Optimal functional capability of engineered T cells may be achievable by the targeted disruption of genes involved in the inhibition of T-cell function and by providing transgenes that encode immune-enhancing molecules (Figure 4). Optimal function may be particularly important in solid cancer and lymphoma, where the tumor microenvironment may be low in amino acids, glucose, and oxygen and rich in immune-suppressive cytokines such as transforming growth factor β.71 Nutrient depletion and transforming growth factor β signaling can lead to the inhibition of mammalian target of rapamycin complex 1 (mTORC1) signaling and thus impair T-cell activation. Although small-molecule drugs such as rapamycin have been used to inhibit mTORC1 signaling, genetic strategies can be used to achieve enhanced mTORC1 function. For example, forced expression of Ras homolog enriched in brain in engineered T cells has led to the upregulation of mTORC1 signaling, which in an experimental murine tumor model has improved elimination of established tumors following adoptive T-cell therapy.72
The delivery of immune stimulatory cytokines is another strategy that has been used to enhance the antitumor effect of engineered T cells. The most studied cytokine is interleukin 12 (IL-12), which was controlled by an NFAT promoter to achieve IL-12 production following TCR engagement.73 Such TCR-controlled expression can potentially overcome the substantial toxicity that is seen when IL-12 is given systemically. However, recent results of a clinical trial revealed toxic side effects of engineered T cells expressing IL-12 under the control of the NFAT promoter.73 Thus, the experience to date has shown that IL-12 is effective in enhancing antitumor activity of adoptive T-cell transfer, but cytokine-mediated toxicity remains a major challenge. The regulated expression of other cytokines and T-cell effector molecules may in the future enhance tumor protection in the absence of systemic side effects (Figure 4).
Conclusions
Exciting research developments combined with impressive clinical results have triggered enthusiasm for T-cell engineering in the academic, clinical, and commercial sectors. Although rapidly evolving tools enable complex genetic modifications to optimize T-cell function, the most important aspect remains the selection of the best target antigens. Although mutated cancer genes are conceptually attractive, they are rarely shared between cancers of the same tissue type, and they may not be presented effectively for TCR recognition. In contrast, a recent study of HLA-presented peptides in CLL revealed a group of 49 tumor-associated self-proteins that provide shared TCR epitopes in many patients with this type of malignancy.74 Therefore, TCRs for shared tumor-associated and linage-specific antigens remain attractive therapeutics for the treatment of hematologic malignancies.
Acknowledgments
The authors thank Duncan Gordon-Smith for assisting with the illustrations.
This work was supported by a program grant from Bloodwise and funding from the Medical Research Council, European Framework Program (EU-FP7), Biomedical Research Centre, Experimental Cancer Medicine Centre, and Wellcome Trust.
Authorship
Contribution: H.J.S. and E.C.M. wrote the manuscript.
Conflict-of-interest disclosure: H.J.S. is scientific advisor and shareholder at Cell Medica. The remaining author declares no competing financial interests.
Correspondence: Hans J. Stauss, Institute of Immunity and Transplantation, Royal Free Campus, University College London, Rowland Hill St, London NW3 2PF, United Kingdom; e-mail: h.stauss@ucl.ac.uk.