In this issue of Blood, Ettinger et al show that the T-cell response to therapeutic factor VIII (FVIII) in a patient with severe hemophilia A due to a multi–exon F8 deletion was oligoclonal and directed against a single epitope located in the C2 domain of the molecule. They further demonstrated that the HLA-DRB1*01:01-restricted T-cell epitope was recognized by the T cells of 2 other hemophilia A patients with the A2201P missense mutation.1
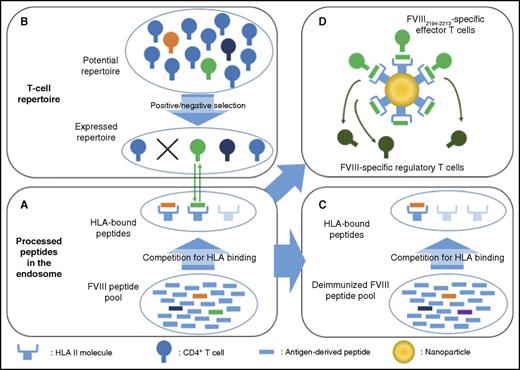
Monoepitopic and oligoclonal T-cell responses to therapeutic FVIII. (A) The immune response to FVIII requires antigen internalization by APCs, processing in endosomes, and FVIII-derived peptides binding to HLA for presentation at the cell surface. A competition for binding to HLA thus occurs within the intracellular pool of FVIII-derived and non-FVIII-derived peptides. (B) For the immune response to develop, and leaving aside the requisite for danger signals that allow the maturation of APCs, there is a need for an appropriate T-cell repertoire. The expressed T-cell repertoire is shaped by positive and negative selection in the thymus, and encounter of antigens at the periphery. It is thus incomplete and may lack critical epitope specificities. In our example, the immunodominant green and orange FVIII peptides outrange other peptides, such as the subdominant dark-blue peptide, for HLA binding, and are exposed at the surface. The potential T-cell repertoire has been selected: T cells specific for the orange peptide have been eliminated, whereas that specific for the green peptide have been retained, thus allowing initiation of a T-cell–mediated anti-FVIII immune response. Presumably, the immune system of a patient with severe hemophilia A without circulating FVIII is not educated against FVIII, and the expressed repertoire of FVIII-reactive T cells has not been deleted. (C) Deimmunization of FVIII would rely on the modification of dominant T-cell epitopes (eg, green epitope changed into purple). The FVIII would then be “invisible” to T cells, provided that subdominant epitopes are not presented by HLA in the absence of the dominant epitope. (D) Nanoparticles coated with MHCII-bound FVIII immunodominant epitope(s) could trigger the generation and expansion of antigen-specific type 1 regulatory CD4+ T cells.
Monoepitopic and oligoclonal T-cell responses to therapeutic FVIII. (A) The immune response to FVIII requires antigen internalization by APCs, processing in endosomes, and FVIII-derived peptides binding to HLA for presentation at the cell surface. A competition for binding to HLA thus occurs within the intracellular pool of FVIII-derived and non-FVIII-derived peptides. (B) For the immune response to develop, and leaving aside the requisite for danger signals that allow the maturation of APCs, there is a need for an appropriate T-cell repertoire. The expressed T-cell repertoire is shaped by positive and negative selection in the thymus, and encounter of antigens at the periphery. It is thus incomplete and may lack critical epitope specificities. In our example, the immunodominant green and orange FVIII peptides outrange other peptides, such as the subdominant dark-blue peptide, for HLA binding, and are exposed at the surface. The potential T-cell repertoire has been selected: T cells specific for the orange peptide have been eliminated, whereas that specific for the green peptide have been retained, thus allowing initiation of a T-cell–mediated anti-FVIII immune response. Presumably, the immune system of a patient with severe hemophilia A without circulating FVIII is not educated against FVIII, and the expressed repertoire of FVIII-reactive T cells has not been deleted. (C) Deimmunization of FVIII would rely on the modification of dominant T-cell epitopes (eg, green epitope changed into purple). The FVIII would then be “invisible” to T cells, provided that subdominant epitopes are not presented by HLA in the absence of the dominant epitope. (D) Nanoparticles coated with MHCII-bound FVIII immunodominant epitope(s) could trigger the generation and expansion of antigen-specific type 1 regulatory CD4+ T cells.
The involvement of CD4+ T cells in the initiation and persistence of the anti-FVIII immune response has long been suspected based on clinical observations and studies in preclinical models of hemophilia A. Patients with a history of high-titer inhibitors experienced a loss of FVIII inhibitors after infection with HIV; the loss was associated with the drop in CD4+ T-cell counts and absence of an anamnestic immune response upon rechallenge using FVIII concentrates. In FVIII-deficient mice, abrogation of the cross-talk between T cells and antigen-presenting cells (APCs) using antibodies to CD40 ligand (CD154), CD80, or CD86, or using CTLA4-Ig constructs, prevents the formation of inhibitors to FVIII.
The large size of FVIII, however, has hampered studies on the specificity of the immune response to FVIII. The molecule encompasses 2332 amino acids (1383 amino acids in its activated form), providing multiple potential T-cell epitopes. Indeed, >1704 FVIII-derived peptides are predicted as core binding peptides for at least one of the 10 most common HLA-DRB1 alleles (Anastas Pashov, Bulgarian Academy of Science, Sofia, e-mail, 10 August 2016). Early work studied the proliferation and cytokine secretion profiles of CD4+ T lymphocytes from patients and healthy donors stimulated in vitro with FVIII or FVIII-derived peptides.2 Strikingly, no clear difference was found in T-cell responses among healthy individuals, hemophilia A patients with neutralizing antibodies (Nab), or hemophilia A patients without Nab. Moreover, proliferation was found against synthetic peptides from all domains of FVIII. This finding was at odds with the expected role of CD4+ T lymphocytes in the anti-FVIII immune response and meant that future studies should also take into account the enormous diversity of hemophilia-causing genetic abnormalities (large/small deletions, intron inversions, missense/nonsense mutations, small deletions/insertions, splice site mutations).
In keeping with the seminal work by Jacquemin et al,3 the initial characterization of FVIII T-cell epitopes performed by the Pratt laboratory focused on hemophilia A patients with missense mutations with Nab. In such patients, the immune response, at least in its early phase, is directed against the epitope of wild-type therapeutic FVIII that corresponds to the mutated epitope of the patient’s endogenous FVIII. Using major histocompatibility complex II (MHCII) tetramer-guided epitope mapping, Pratt and colleagues isolated and characterized FVIII-specific T-cell clones from patients with the R593C or A2201P mutations4 : the C2 domain-derived peptide 2194-2213 was identified as a dominant HLA-DRB1*01:01-restricted T-cell epitope in 2 brothers with the A2201P mutation.
In the present work, Pratt’s group studied a severe hemophilia A patient with the HLADRB1*01:01/10:10 haplotype and with no trace of circulating endogenous FVIII antigen, who developed anti-FVIII Nab following replacement therapy with exogenous FVIII and failed immune tolerance induction (ITI) protocols (ie, desensitization of the patients with high-dose FVIII). In such patients, the T-cell immune response would be expected to target multiple FVIII epitopes and to involve different T-cell clones (ie, polyclonal). This supposition derives from the large number of predicted T-cell epitopes in therapeutic FVIII (175 in the case of DRB1*01:01), by experiments on HLA-DR transgenic mice5 and by MHC-associated peptide proteomics on FVIII-loaded human monocyte-derived dendritic cells.6 Surprisingly, the FVIII2194-2213 peptide presented on DRB1*01:01 was identified as the unique T-cell epitope in the patient, and the repertoire of FVIII2194-2213 peptide-specific T cells was extremely restricted in terms of usage of TCRB-encoding genes. The T-cell response was thus monoepitopic and oligoclonal.
Confirmation of such an oligoclonal anti-FVIII T-cell response in all severe hemophilia A patients with Nab would represent a drastic paradigm shift in our understanding of FVIII immunogenicity, and, potentially, of the immunogenicity of large therapeutic proteins in general. The results should however be considered with caution. Only peptides from the A2, C1, and C2 domains, that represent 700 amino acids of the FVIII molecule, were tested, leaving open the possibility that additional T-cell epitopes are present in the A1, B, and A3 domains of FVIII. Besides, the patient underwent (and failed) ITI, which means that he has received large amounts of FVIII for a long period of time. It is unclear how such an intensive antigenic stimulation of the patient’s immune system has impacted the population of FVIII-specific CD4+ T lymphocytes, possibly leading to an “epitope shrinking” of the T-cell response. The inclusion of additional Nab-positive patients preferably sampled before initiation of ITI and demonstration that the FVIII T-cell response is also restricted to unique immunodominant epitopes in the case of patients with other HLA-DR alleles are thus warranted.
Nonetheless, confirmation that, for each HLA allele, a single epitope is the target of effector T cells would encourage deimmunization of FVIII by mutation of key residues in the identified immunodominant T-cell epitopes (see figure). The introduced mutations should obviously be compatible with normal hemostatic functions of FVIII, and with its stability and capacity to be exported from the endoplasmic reticulum. One should also ensure that the change in equilibrium within the peptide pool in the endosome does not result in the exposure of subdominant T-cell epitopes (see figure). Tolerance to FVIII has been induced in FVIII-deficient mice using oral, transplacental, or B-cell–mediated delivery of the immunodominant A2 and C2 FVIII domains.7-9 In line with these results, key immunodominant T-cell epitopes could be exploited to specifically eliminate effector T cells or induce antigen-specific regulatory T cells. Alternatively, administration of nanoparticles coated with MHCII/FVIII peptide complexes (see figure) could trigger specific tolerance as demonstrated in experimental models of several human autoimmune diseases.10 Clearly, a paucity of T-cell epitopes in FVIII would change the perspective of treatment of patients with hemophilia A.
Conflict-of-interest disclosure: The authors declare no competing financial interests.