Abstract
BACKGROUND: Advanced age, AML arising from an antecedent hematologic disorder (AHD), and therapy-related AML (t-AML) are well established risk factors associated with poor outcome in AML. This subgroup of AML has been associated with lower rates of complete remission (CR) and shorter disease-free survival compared with de novo AML in younger patients (pts), when using standard therapy. Developing newer strategies to improve outcomes in this subgroup of pts is therefore a critical unmet need. Establishing baseline metrics from which to evaluate newer approaches is important. We sought to review our own experience with pts treated with different therapies at our institution to establish this baseline.
METHODS: We retrospectively reviewed pts aged 60-75 years with newly diagnosed AML treated at our institution from 1990-2015 with one or more of the following characteristics: history of prior MDS, CMML, MPN, or aplastic anemia, the diagnosis of t-AML, or AML with karyotypic abnormalities characteristic of MDS as defined by the WHO. The pts were grouped into 5 study cohorts based on their treatment regimen: 1) High-dose Ara-C based intensive chemotherapy (HiDAC), 2) Hypomethylating agents (HMA)/HMA combinations, 3) Low-dose Ara-C combinations (LDAC), 4) Liposomal Ara-C:Daunorubicin (CPX-351), and 5) Other investigational agents (INV). Pts' karyotypes were divided into 4 categories: adverse karyotype (complex: defined by >2 chromosomal abnormalities, Del 5/5q, Del 7/7q, 11q, 3q abnormality); diploid karyotype, intermediate karyotype (karyotype other than adverse or diploid); and unknown/not available. Endpoints included complete response (CR) rate/CR with low platelets (CRp) and 8-week mortality rates. Both univariate and multivariate analyses were performed to examine the relationships between disease characteristics and outcomes (CR, Overall survival).
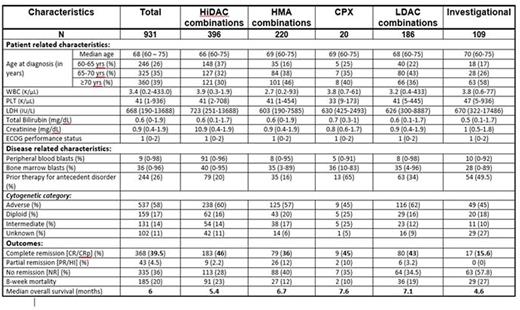
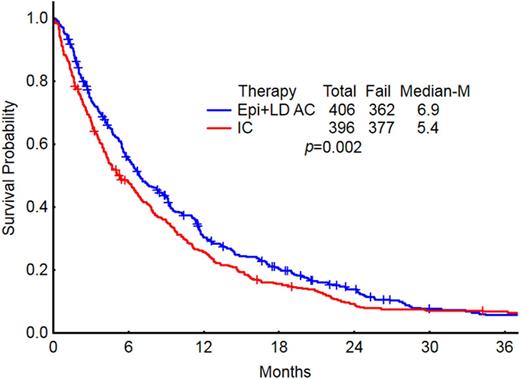
RESULTS: We evaluated a total of 931 pts with newly diagnosed AML meeting these criteria of age and secondary AML (s-AML). The baseline characteristics of pts in each group are summarized in table 1. The median age was 68 (60-75) years. Most pt characteristics were well balanced across the 5 treatment groups. Karyotyping was performed in 89% of the pts: 57.5% belonged to adverse risk profile and 31% belonged to intermediate risk profile (excluding diploid). The overall CR/CRp rates for the entire group was 39.5%. CR/CRp rates were lower in the HMA (36%) and INV (16%) groups compared to HiDAC (46%), CPX (45%) and LDAC (43%). Eight week mortality rate for the entire group was 20%: HiDAC (23%), HMA (12%), CPX (10%), LDAC (19%), and INV (27%). The median OS for the entire group was 6 months and by each treatment regimen is shown in Figure 1. There was a significant difference in OS between the 5 treatment groups. Pts receiving less intensive regimens [HMA + LDAC] had a superior median OS compared to those receiving HiDAC-based regimens (6.9 vs. 5.4; P=0.002, Figure 2). There was no difference in median survival in pts treated with CPX compared with conventional lower-intensity approaches (HMA + LDAC) (p=0.75). In the multivariate analysis, age >70yrs, adverse karyotype, and prior treatment for AHD were associated with a decreased OS. Similarly, use of HMA/INV treatment, adverse/intermediate risk karyotype, history of prior treatment for AHD were associated with lower CR rates.
CONCLUSIONS: The outcomes of older pts with newly diagnosed s-AML are poor and reflective of their advanced age and poor-risk karyotype. Various treatment approaches have been attempted to improve outcomes. Lower-intensity approaches such as HMA and LDAC-based regimens have response rates similar to HiDAC regimens, but are associated with lower early mortality and improved overall survival. While the investigational liposomal formulation CPX-351was associated with favorable CR rates and low early mortality, the median OS was similar to pts treated with low intensity therapy. The unsatisfactory outcomes using other investigational agents in the INV group underscores the need for more effective therapies for these patients.
Baseline study population characteristics and disease outcomes
Baseline study population characteristics and disease outcomes
OS in pts by the treatment regimen group
OS comparison in pts who received IC/HiDAC vs less intensive (LDAC + HMA) therapies
OS comparison in pts who received IC/HiDAC vs less intensive (LDAC + HMA) therapies
Kantarjian:BMS, Pfizer, Amgen, Novartis: Research Funding. Konopleva:Reata Pharmaceuticals: Equity Ownership; Abbvie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Stemline: Consultancy, Research Funding; Eli Lilly: Research Funding; Cellectis: Research Funding; Calithera: Research Funding. DiNardo:Celgene: Research Funding; Abbvie: Research Funding; Novartis: Other: advisory board, Research Funding; Daiichi Sankyo: Other: advisory board, Research Funding; Agios: Other: advisory board, Research Funding. Jain:Celgene: Research Funding; Infinity: Research Funding; ADC Therapeutics: Consultancy, Honoraria, Research Funding; Novimmune: Consultancy, Honoraria; Incyte: Research Funding; Abbvie: Research Funding; Novartis: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Genentech: Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; BMS: Research Funding; Seattle Genetics: Research Funding. Wierda:Acerta: Research Funding; Novartis: Research Funding; Gilead: Research Funding; Abbvie: Research Funding; Genentech: Research Funding. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.