Abstract
Background Overall survival (OS) of lower-risk myelodysplastic syndrome (LR-MDS) patients treated with red blood cell transfusions (RBCT) is inferior to that of untransfused patients. RBCTs are associated with iron toxicity. Therefore, many transfused LR-MDS patients receive iron chelation according to (inter)national guidelines. The value of iron chelation in LR-MDS remains unproven. The aim of this study is to assess in a prospective, observational setting the efficacy of iron chelation to counteract the effects of iron overload in LR-MDS.
MethodsThree iron chelators are available in Europe for treatment of iron overload, but availability varies from country to country. We first assessed the impact of treatment of the 3 iron chelators on survival among the 195 patients treated with iron chelation. Secondly, we developed a model of a contemporary, observational control within the EU-MDS registry of patients who met the inclusion criteria, but who did not receive iron chelation.
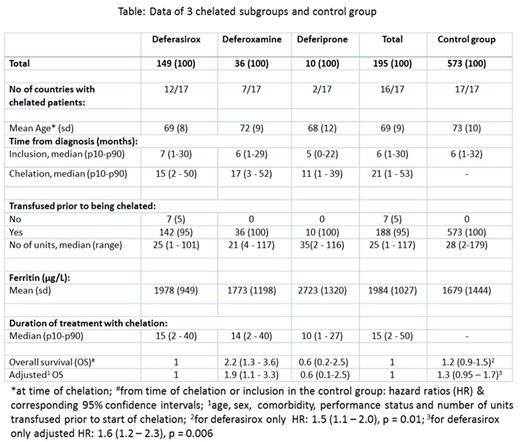
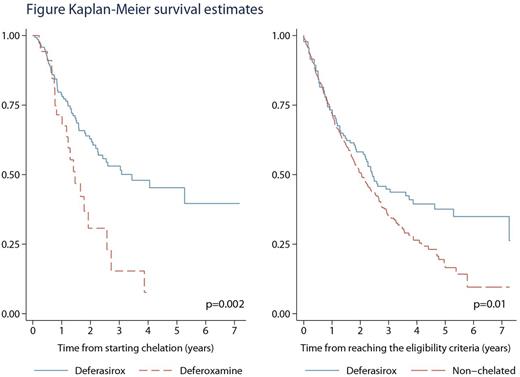
ResultsThe EUMDS registry has accrued 2084 patients as of July 21, 2016. At this point, 195 patients had received chelation therapy (table). 82 (42%) Patients had died (22 after progressing to AML) and 34 alive patients had progressed to AML. The median time on chelation for all 195 patients was 15 months. Still-living patients had a median time on chelation of 18 months. Of the chelated patients, 149 received deferasirox as the initial chelator, 36 deferoxamine and 10 deferiprone. Treatment duration of the 3 different chelators and the use of the 3 chelators per country is given in table. Twenty patients switched from one chelator to another, but usually the treatment period of the 2nd chelator was short compared to the treatment period of the 1st chelator (data not shown). The Kaplan-Meier estimate showed a significantly better OS for the 149 patients initially treated with deferasirox compared to the 36 patients treated with desferoxamine (log rank p = 0.0021). Multivariate analysis of the 2 groups showed a hazard ratio (HR) of 2.2 (95% CI: 1.3-3.6) and after adjustment a HR of 1.9 (95% CI: 1.1-3.3). We compared the outcome of a non-chelated control group of 573 transfusion dependent patients with 192 chelated patients who achieved one of the following inclusion criteria, recommended to start iron chelation according to (inter)national guidelines: >15 units of RBCT or >1 unit/month during a 6-month period between visits or ferritin >1000 mg/L. Patients were analysed from time of reaching the criteria using receipt of chelation as the time-varying covariate. The unadjusted HRs and 95% CIs were: 1.2 (0.92 - 1.5) and 1.3 (0.95 - 1.7) after adjustment for relevant factors (p = 0.10). The corresponding risk estimates for the analyses restricted to the 149 patients initially treated with deferasirox were 1.5 (1.1 - 2.0) and 1.6 (1.2 - 2.3) respectively (p = 0.006).
InterpretationClinical practice of iron chelation in LR-MDS varied considerably in the 17 European countries participating in the EUMDS Registry. The most frequently used chelator is deferasirox. Use of this chelator varied from 0% to 25% per country. OS was significantly better after treatment with deferasirox when compared to the classical chelator deferoxamine. OS was also significantly better when compared with a large control group of 573 patients, even after adjustment for all relevant prognostic factors.
Fenaux:Celgene, Janssen, Novartis, Astex, Teva: Research Funding; Celgene, Novartis, Teva: Honoraria. Symeonidis:Genesis: Honoraria; Roche: Honoraria; Amgen: Honoraria; Takeda: Consultancy, Honoraria. Almeida:Alexion: Speakers Bureau; BMS: Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Shire: Speakers Bureau; Celgene: Consultancy, Research Funding, Speakers Bureau. Savic:Novo Nordisk: Other: Investigator. de Witte:Incyte: Consultancy; Celgene: Consultancy; Novartis: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract