Abstract
Introduction: The genetic basis of several acute myeloid leukemia (AML) subtypes remains poorly characterized, such as that of acute erythroid leukemia (AEL, AML M6) which is currently subclassified by morphology alone, and is associated with poor outcome. Here we sought to perform a definitive genomic analysis of AEL and translate these findings into faithful experimental models and novel therapeutic approaches.
Methods: We studied 151 AEL cases (19.2% pediatric, 4.6% young adult, 21.9% adult and 54.3% older adult). Diagnosis of AEL was centrally confirmed and subclassified according to WHO 2008 and revised 2016 criteria. Whole exome and/or genome sequencing, RNA-sequencing and SNP array analysis were performed on all cases and in 2 AEL cell lines (TF-1 and Hel). Genomic data were compared to those from non-M6 childhood and adult AML from TARGET (n=192) and TCGA (n=197) studies. The functional effects of fusion transcripts and mutated genes were examined in IL-3 dependent Ba/F3 cells, NIH3T3 cells for focus formation assays and/or mouse lineage negative hematopoietic stem cells (lin- HSC) for colony forming and transplantation assays. Avatars of human AEL were established in immunocompromised NSGS and MISTRG mice for preclinical studies.
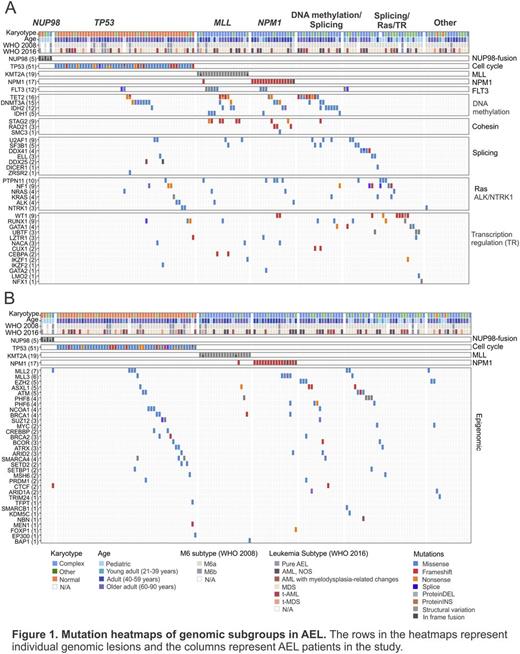
Results: a) Genomic landscape of AEL. We identified 2,250 non-synonymous clonal and subclonal somatic mutations in 1,723 genes with a mean of 16.4 per case (range 2-88) and with missense and frameshift mutations accounting for 47.1% and 22.5% of all mutations, respectively. 78 genes were recurrently mutated in at least 3 cases. In frame fusions were detected in 31% of childhood and 27.5% of adult cases, and were more frequent in cases with complex karyotype. 124 potential driver genes were identified by statistical analysis or known pathogenic role in cancer, 9 of which were recurrent novel targets of mutation, most commonly involving chromatin modification (60.3%), cell cycle/tumor suppression (TP53, 33.8%), DNA methylation (28.5%), transcription regulation (26.5%), splicing (15.9%), NPM1 (11.9%), Ras (11.3%), JAK-STAT signaling (9.9%), the cohesin complex (8.6%), ALK/NTRK1 (4.6%) and PI3K signaling (3.3%). Overall, 33% of cases harbored a mutation in signaling genes amenable to inhibition by tyrosine kinase/Ras inhibitors. Mutations in TP53 and DNA methylation genes were significantly more frequent in adults while mutations in transcription regulators and Ras pathway were more frequent in children. Splicing mutations correlated with MDS phenotype and PI3K alterations with therapy-related AEL. Based on the co-occurrence and exclusivity of mutations 7 main distinct AEL genetic subtypes were defined: 1) pediatric AEL with NUP98-rearrangements (3.3% of all cases); 2) adult complex karyotype AEL with TP53 mutations (33.8%); 3) AEL with MLL-rearrangements (12.6%); 4) NPM1-mutated AEL (11.9%); 5) DNA-methylation/splicing mutated AEL (17.8%); 6) splicing/Ras/transcription regulation mutated AEL (21.2%) and 7) Other (8.6%) (Fig.1A). Mutations of chromatin modifiers occurred independently of karyotype, age and subtype (Fig.1B). NUP98-fusions and mutations in PTPN11, UBTF and GATA1 were more frequent in pediatric AEL compared to non-M6 AML. Among adults, mutations in TP53 and MLL were more frequent in AEL while FLT3, NPM1, DNMT3A and IDH1 were more in frequent in non-M6 subtypes. A complex karyotype, therapy-related AEL, TP53 mutations and NUP98-rearrangements were associated with poor outcome.
b) Functional AEL modeling and therapeutic translations. Expression of NUP98-JARID1A in lin- HSC resulted in sustained self-renewal and development of an aggressive transplantable leukemia. At least three classes of signaling pathway mutations are targetable in AEL. ALK mutations in the extracellular MAM domain transformed Ba/F3 cells which were sensitive to crizotinib in vitro. Mutations in the tyrosine kinase domain of NTRK1 transformed NIH3T3 cells and were sensitive to entrectinib in vitro. Targeting of JAK-STAT, mTOR and PI3K pathways were examined in xenografts and sensitivity to JAK2 inhibitor ruxolitinib was confirmed in vivo.
Conclusions: We provided the first comprehensive landscape of genomic alterations in AEL and defined distinct genomic groups with unique patterns of mutation occurrence compared to non-M6 AML. Finally, we showed that several pathogenic pathways are amenable to inhibition by approved targeted compounds.
Meggendorfer:MLL Munich Leukemia Laboratory: Employment. Wei:Novartis: Honoraria, Research Funding. Loh:Abbvie: Research Funding; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Haferlach:MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Mullighan:Amgen: Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees; Loxo Oncology: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.