Abstract
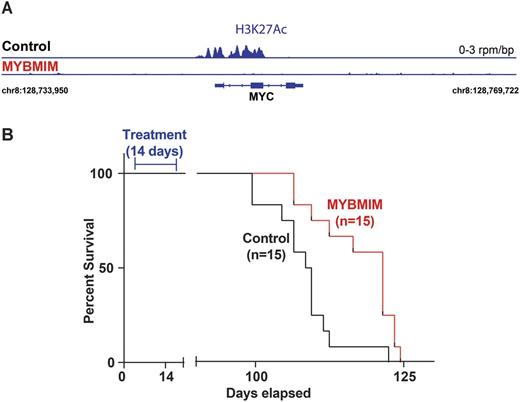
Aberrant gene expression is a hallmark of acute leukemias. However, therapeutic strategies for its blockade are generally lacking, in large part due to the pharmacologic challenges of drugging transcription factors. MYB-driven gene trans-activation with CREB-binding protein (CBP) is required for the initiation and maintenance of a variety of acute lymphoblastic and myeloid leukemias, including refractory MLL-rearranged leukemias. Using structure-guided molecular design, we developed a prototypical peptidomimetic inhibitor MYBMIM that interferes with the assembly of the molecular MYB:CBP complex at μM concentrations and rapidly accumulates in the nuclei of AML cells. We found that treatment of AML cells with MYBMIM, but not with its inactive near-isosteric analogue TG3, led to the displacement and dissociation of MYB:CBP complex in cells, causing rapid downregulation of MYB-dependent gene expression including MYC and BCL2 oncogenes. This was associated with obliteration of H3K27Ac-driven oncogenic enhancers induced by CBP and enriched for MYB binding sites (Figure 1A; p=1e-118). Both human MLL-rearranged and non-rearranged AML cells, but not normal CD34+ umbilical cord blood progenitor cells, underwent sustained mitochondrial apoptosis in response to MYBMIM treatment, an effect that could be partially blocked by ectopic expression of BCL2. We observed that MYBMIM treatment (50 mg/kg/day) impeded leukemia progression and extended survival of immunodeficient mice engrafted with primary patient-derived MLL-rearranged leukemia cells (Figure 1B; p=3.8e-3). These findings emphasize the exquisite dependence of human AML on MYB:CBP transcriptional dysregulation, and establish a pharmacologic approach for its therapeutic blockade.
MYBMIM blocks oncogenic gene expression (A) and impedes leukemogenesis in vivo(B).
MYBMIM blocks oncogenic gene expression (A) and impedes leukemogenesis in vivo(B).
Armstrong:Epizyme, Inc: Consultancy; Vitae Pharmaceuticals: Consultancy; Imago Biosciences: Consultancy; Janssen Pharmaceutical: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.