Abstract
Background: CPX-351 is a liposomal formulation of cytarabine and daunorubicin encapsulated at a synergistic 5:1 molar ratio. Previous studies (including a pivotal phase 3 for pts with secondary AML) have shown lesser toxicity, higher response rates and improved overall survival (OS) with CPX-351 compared to 3+7 regimen, especially among poor risk and secondary AML patients. Phase I and II studies have shown complete responses (CR) occurring at doses below the maximum tolerated dose (MTD; i.e 100 U/m2), suggesting that 75 and 50 U/m2 doses used as induction therapy could be effective and potentially better tolerated in AML patients considered at high risk (30-50%) for 60-day mortality. We herein report interim results from a randomized open-label, single institution, phase II study of first-line CPX-351 in patients with AML at high risk of induction therapy (NCT 02286726).
Methods: Pts with newly diagnosed AML considered at high risk for induction mortality defined as having at least 1 (if ≥60 years) or 2 (if <60 years) of the following risk factors: 1) AML-related factors including previous MDS, CMML, or MPD; history of exposure to cytotoxic chemotherapy (t-AML)), or WHO-defined AML with MDS-related changes or de novo AML with MDS-associated karyotype; unfavorable cytogenetics as defined by the European Leukemia Net; 2) patient-related factors including age ≥70, ECOG performance status (PS) ≥2, and serum creatinine >1.3 g/dL. This is a 2- or possibly 3-arm trial with one study arm receiving 50 U/m2 and the other 75 U/m2 of CPX-351 on days 1, 3, 5 for induction. If no excess toxicity is identified at 75 U/m2, a 3rd arm using 100 U/m2 is to be opened. The primary objective is to evaluate efficacy (determined by the rate of CR+CRi) for each arm. Other endpoints include the rate of dose limiting toxicities (DLTs) (comprising induction mortality at day 60), OS and event free survival (EFS). For each arm, CR/CRi and DLTs rates will be estimated with 95% confidence intervals (CI). Fisher's exact test will compare response and toxicity rates between the two dose levels.
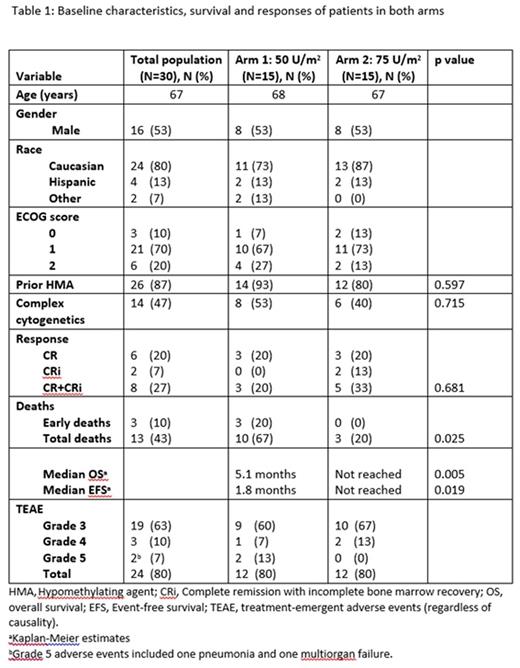
Results: 30 patients were randomized in 1:1 ratio to CPX-351 50 U/m2 or 75 U/m2. The median age was 67 years (range: 54-83). The 2 study arms were well balanced for sex, race, age, PS, cytogenetics and prior therapy with hypomethylators (Table 1). All patients were evaluable for response and toxicity. The CR+CRi rate was not significantly different between the 2 arms (20% vs 33.33%, P=0.681); the higher-dose arm resulted in superior OS (HR=0.202; p=0.005; 95% CI (0.06-0.62); median OS 5.1 mo vs not reached) and EFS (HR=0.25; p=0.019; 95% CI (0.08-0.75); median EFS 1.8 mo vs not reached). 60-day mortality favored the 75 units/m2 dose of CPX-351 (20% vs. 0%) Grade 3-5 treatment-emergent adverse events were similar and equal for the 2-dose levels (80%) and there have been no treatment-related deaths and no DLTs in either arm.
Conclusion: In AML patients at high risk of induction mortality, the 2 sub-MTD dose levels of CPX-351 (50 and 75 U/m2) appear safe and show clinical benefit. Since there appears to be equal or better clinical benefit with 75 U/m2 without an increase in 60-day mortality, the third arm testing the MTD (100 U/m2) in this high risk group will open. Clinical trial information: NCT02286726
Konopleva:Calithera: Research Funding; Cellectis: Research Funding. DiNardo:Daiichi Sankyo: Other: advisory board, Research Funding; Abbvie: Research Funding; Agios: Other: advisory board, Research Funding; Celgene: Research Funding; Novartis: Other: advisory board, Research Funding. Daver:Kiromic: Research Funding; Karyopharm: Honoraria, Research Funding; Ariad: Research Funding; Sunesis: Consultancy, Research Funding; BMS: Research Funding; Otsuka: Consultancy, Honoraria; Pfizer: Consultancy, Research Funding. Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy. Wierda:Acerta: Research Funding; Novartis: Research Funding; Gilead: Research Funding; Genentech: Research Funding; Abbvie: Research Funding. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.