Abstract
The functional or genetic inactivation of p53 hampers human tumor treatment. Therefore, novel therapeutic strategies are needed. ONC201 is a p53-independent inducer of apoptosis that is the founding member of the imipridone class of novel anti-cancer compounds, which possess a unique pharmacophore. We discovered that ONC201 exerts anti-tumor effects via ATF4 induction through activation of an atypical integrated stress response (ISR) (Ishizawa et al. and Kline et al, Sci Signal, 2016). Several clinical trials of ONC201 are ongoing in advanced cancers, showing a promising safety profiling and signs of clinical activity in both solid tumors and hematopoietic malignancies. In this study, we investigated the effects of ONC212, which has emerged as a highly potent member of the imipridone family, in preclinical models of hematological malignancies.
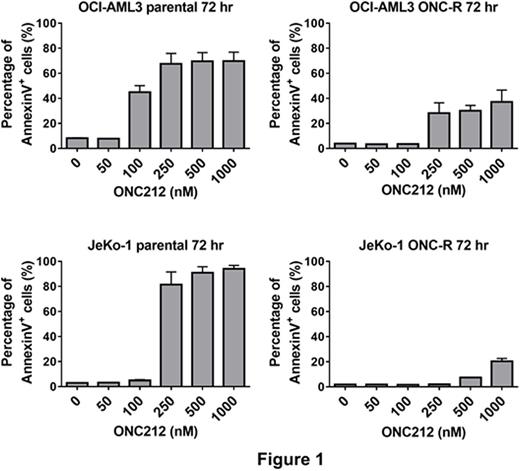
ONC212 exerted potent and prominent apoptogenic effects on acute myeloid leukemia (AML) and mantle cell lymphoma (MCL) cell lines (e.g., ED50s of 141.0 nM in p53 wild-type OCI-AML3 cells, 105.7 nM in MOLM13 cells, and 265.2 nM in p53-null JeKo-1 cell lines). Time course analysis of apoptosis in OCI-AML3 cells showed that ONC212 takes more than 36 hours to start to induce apoptosis, which is similar to observations with ONC201. Next, we further examined similarities between ONC212 and ONC201 by evaluating the in vitro efficacy of ONC212 in ONC201-resistant (ONC201-R) cell lines that we have generated by chronic exposure of MCL and AML cell lines to ONC201, of which ED50s for ONC201 treatment at 72 hrs were all > 5 μM. Interestingly, the ONC201-R cell lines were more resistant to ONC212 than the isogenic ONC201-naïve cells (Figure 1), indicating that these cell lines are cross-resistant to ONC212.
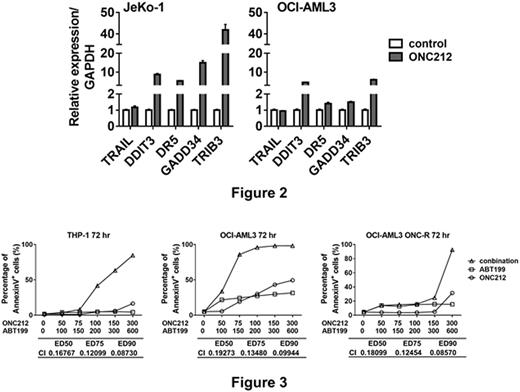
We previously proved that increased protein translation of the transcription factor ATF4 is one of the major molecular events involved in ONC201-induced apoptosis (Ishizawa et al., Sci Signal, 2016). Similarly, ATF4 protein abundance was increased by 24-hour treatment with ONC212. DDIT3 (CHOP) gene, a target of ATF4, was transcriptionally upregulated in parallel with its target genes GADD34, DR5 and TRIB3 in ONC212-treated JeKo-1 and OCI-AML3 cells by 24 hrs after treatment (Figure 2). Of note, ONC201 was reported to transcriptionally induce TRAIL in a p53-independent manner in solid tumors (Allen et al., Sci Transl Med, 2013), but it was not operational in hematological cell lines (Ishizawa et al., Sci Sig 2016). Consistently, we also confirmed that ONC212 does not increase TRAIL mRNA in MCL (JeKo-1) and AML (OCI-AML3) cells.
BCL-2 is a protective factor for cells under endoplasmic reticulum stress, which is one way to activate ISR. Therefore, we investigated whether the BCL-2 inhibitor ABT-199 sensitizes hematopoietic malignant cells to ONC212. Apoptosis was significantly higher in the combination than either drug alone in MCL and AML cell lines even in THP-1 and OCI-AML3 cells that are relatively resistant to ONC201/212 and/or ABT-199 (Figure 3), suggesting that this combination could overcome the resistance to either of agents. Indeed, the combination was also synergistic in the OCI-AML3 ONC201-R cell line (Figure 3).
Taken together, our preclinical studies suggest that ONC212 is a promising and potent new member of the impridone class of anti-cancer compounds that warrants further development in hematological malignancies. The combination of ONC212 with ABT-199 is attractive, considering that acquired resistance after a short-term response remains a clinical challenge with ABT-199.
Konopleva:AbbVie: Research Funding; Genentech: Research Funding. Allen:Oncoceutics Inc.: Employment. Stogniew:Oncoceutics Inc.: Employment, Equity Ownership. Andreeff:Oncoceutics Inc.: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.