Abstract

Background: The outcome of patients (pts) with myelodysplastic syndrome (MDS) after hypomethylating agent (HMA) failure is poor with a median overall survival (OS) of only 4-6 months; there is no standard of care in this setting. Omacetaxine mepesuccinate (OM) has previously been shown to be safe and effective in acute myeloid leukemia (AML), including in pts who progressed from MDS. We sought to evaluate the safety and efficacy of OM in pts with MDS after HMA failure.
Methods: Adult pts with MDS or CMML who had not responded to, progressed on, or relapsed after HMA therapy were eligible for this study. Pts were required to be intermediate-2- or high-risk by IPSS or be classified as RAEB-1 (5-9% BM blasts), RAEB-2 (10-19% BM blasts) or CMML (5-19% BM blasts). Pts received OM 1.25 mg/m2 SQ every 12 hours x 3 days on a 28-day cycle. The primary efficacy outcomes were overall survival (OS) and the overall response rate (ORR), defined as the composite of complete remission (CR), partial remission (PR), marrow CR (mCR) and hematologic improvement (HI). Secondary outcomes included the remission duration, relapse-free survival (RFS), and the safety profile of the regimen.
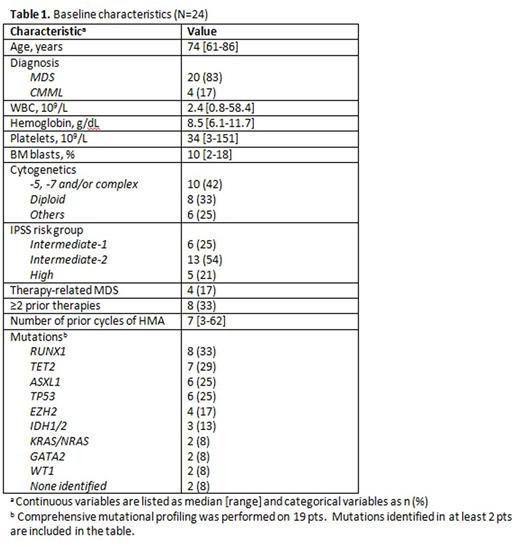
Results: Between 6/2015 and 7/2016, 24 pts have been treated. Baseline characteristics are summarized in Table 1. The median age of the cohort was 74 years (range, 61-86 years). The median BM blasts was 10% (range, 2-18%) and 10 pts (42%) had adverse karyotype. IPSS risk group was intermediate-1 in 6 pts (25), intermediate-2 in 13 (54%), and high in 5 (21%). Among 19 pts who underwent comprehensive mutation profiling, 17 (89%) had at least one molecular mutation identified. The most frequently identified mutations were RUNX1 in 8 pts (33%), TET2 in 7 (29%), ASXL1 in 6 (25%), TP53 in 6 (25%), and EZH2 in 4 (17%).
The median duration of follow-up was 4 months (range, 1-13 months), and the median number of cycles of OM received was 3 (range, 1-10). The ORR was 29%; all 7 pts who responded to OM achieved mCR as best response; no pts achieved CR or HI. The median time to best response was 1.9 months (range, 1.1-5.7 months). Responses were seen across cytogenetic risk groups: 4 responders were diploid, 1 had complex karyotype, 1 had monosomy 7 and 1 had trisomy 8. The response rates for pts with diploid and adverse-risk cytogenetics were 50% and 20%, respectively (P=0.18). Of the 7 responders, 3 remain on study, 2 relapsed, and 2 died while on study. To date, 8 pts (33%) have died. The median OS was 8.2 months, and the 6-month OS rate was 67%. The median OS for pts with diploid and adverse-risk cytogenetics was not reached and 8.2 months, respectively (P=0.08). The median RFS was 3.4 months, and the 6-month RFS rate was 33%. Among the 2 pts who relapsed, the remission duration was 3.0 and 3.4 months. AML developed in 8 pts (33%) with a median time to AML of 2.3 months (range, 0.9-3.9 months).
Treatment was overall well-tolerated. Among 17 pts who have received ≥2 courses of OM, 2 (12%) required dose reduction during subsequent cycles. Most adverse events were grade 1-2; grade ≥3 adverse events occurred in 8 pts (33%). The most frequent non-hematologic adverse events were fatigue in 12 pts (50%), nausea in 7 (29%), infection in 6 (25%), mouth sores in 5 (21%), and headache in 3 (13%). Five pts died while on study; the causes of death were sepsis/pneumonia (n=3), pneumonitis (n=1) and unknown cause (n=1).
Conclusions: In pts with MDS who previously failed HMA therapy, OM was safe and resulted in an ORR of 29%. The median OS of 8.2 months compares favorably to the historical reports of survival post-HMA failure.
Konopleva:Cellectis: Research Funding; Calithera: Research Funding. DiNardo:Daiichi Sankyo: Other: advisory board, Research Funding; Novartis: Other: advisory board, Research Funding; Abbvie: Research Funding; Celgene: Research Funding; Agios: Other: advisory board, Research Funding. Cortes:ARIAD: Consultancy, Research Funding; Bristol-Myers Squib: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding. Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract