Abstract
Retinoic acid (RA), a metabolite of vitamin A, modulates a variety of aspects of the immune system, primarily because of its diverse effects on a wide range of immune cells. Initiation of RA-mediated transcription requires the binding of RA to heterodimeric nuclear receptors composed of RA receptors (RARα, β, and γ) and retinoid X receptors (RXRα, β, and γ). Although RA signaling is tolerogenic under steady state conditions, previous studies, including ours, have shown that RA can enhance pro-inflammatory responses in acute graft-versus-host disease (aGVHD) (Blood. 2013; 122(12): 2125). In that study, we demonstrated that donor T cells expressing a dominant negative RARα (DNRARα) markedly impaired GVHD lethality capacity. This paradoxical function in aGVHD was attributed to excessive RA production caused by the upregulation of RA-synthesizing enzymes, retinaldehyde dehydrogenases (RALDH), in hematopoietic and non-hematopoietic cells. In the current study, we investigated genetic and translational approaches to ablate RA synthesis and signaling as a means to treat aGVHD.
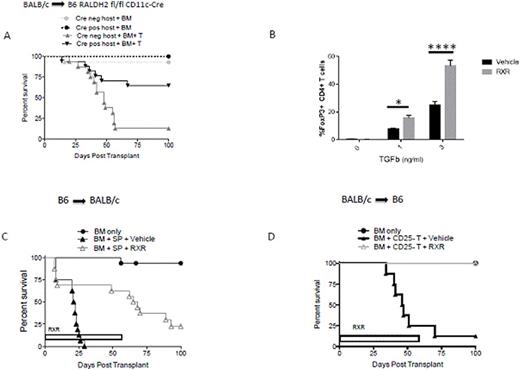
The 4 isoforms of the RALDH enzyme are differentially expressed in a variety of cell types. RALDH-2 is the predominant isoform in dendritic cells (DCs), whereas RALDH-1 levels were found to be higher in intestinal epithelial cells (IECs) during aGVHD. We hypothesized that conditional deletion of either RALDH-2 in host CD11c+ DCs, or RALDH-1 in host IECs using a Cre-Lox system would diminish RA synthesis and reduce aGVHD. Consistent with this hypothesis, ablation of RA synthesis by RALDH-2 in host CD11c+ DCs significantly reduced aGVHD (Figure 1A; p <0.0001) in a fully MHC mismatched BALB/c (H-2d) into C57BL/6 (B6, H-2b) mouse model of aGVHD. However, deletion of RALDH-1 in IECs failed to attenuate aGVHD (not shown), which could be attributed to the existence of other isoforms in these cells. To circumvent the potential contribution of multiple RA synthesizing isoforms, we generated mice over expressing CYP26A1, a key RA catabolizing enzyme. Over expression of CYP26A1 in host hematopoietic cells using CYP26A1stop/stop-VAV-Cre recipients significantly mitigated aGVHD (p<0.005; not shown), providing novel insights into the tissue-specific role of RA signaling in aGVHD.
We further explored RA signaling in aGVHD using a translational approach. IRX4204 is a novel and highly specific RXR agonist currently in clinical trials for autoimmune diseases, which can sequester RXR receptors and phenocopies the genetic approach using DNRARα T cells and allowing translation into the clinic. We discovered that IRX4204 can enhance in vitro CD4+CD25+Foxp3+ Regulatory T cell (iTreg) generation from naïve CD4+Foxp3- precursors (Figure 1B), suggesting it might increase Treg in vivo, and therefore be therapeutically useful for aGVHD prevention. To determine the effects of IRX4204 in aGVHD, we used two fully MHC mismatched mouse models: (B6 (H-2b) into BALB/c (H-2d) and BALB/c (H-2d) into B6 (H-2b). Recipients treated with IRX4204 had significantly prolonged survival time (Figure 1C&D), reduced weight loss, and better clinical scores compared to vehicle-treated mice. IRX4204 also significantly reduced donor T cell proliferation, effector differentiation and a 2-4 fold reduced production of pro-inflammatory cytokine (IFN-γ, TNF-α). Despite up-regulating gut-homing receptors on donor T cells, intestinal (and liver) GVHD pathology was reduced, which was associated with higher IEC integrity, assessed by po FITC-dextran serum levels. Compared to controls, IRX4204-treated recipients had a higher frequency of Treg in the spleen, mesenteric lymph nodes (p <0.0001) and colon (p <0.001). To determine whether IRX4204 mitigated aGVHD by promoting peripheral Treg (pTreg) generation, we utilized Treg-depleted donor T cells from either wild type (WT) or Scurfy (Sc) mice in our B6 into BALB/c GVHD model. Sc mice have a FoxP3 gene deletion and therefore cannot produce pTreg. Interestingly, IRX4204 attenuated aGVHD only in recipients that were given WT T cells, indicating that the generation of pTregs was critical for IRX4204-mediated aGVHD protection. Overall, these data lay a strong foundation for the development of novel drug based therapies for aGVHD and other inflammatory disorders by ablating RA synthesis and signaling.
Chandraratna:Io Pharmaceuticals: Other: Employed by Io Pharmaceuticals and is a board member, holding the titles of title of President and Chief Scientific Officer, and has ownership interest in Io Pharmaceuticals. .
Author notes
Asterisk with author names denotes non-ASH members.