Abstract
Background: Burkitt leukemia/lymphoma is a highly aggressive B-cell neoplasm that is potentially curable with chemoimmunotherapy. While patients (pts) who are refractory to initial therapy or subsequently relapse are considered to have a poor prognosis, few studies have systematically evaluated the outcomes of these pts.
Methods: We retrospectively identified 146 adult pts with Burkitt or Burkitt-like leukemia or lymphoma treated at our institution between 1992 and 2013 with frontline hyper-CVAD-based regimens. 12 pts died before initial response assessment and 4 were unevaluable due to inadequate records; these pts were therefore excluded from the analysis. Among the 130 pts evaluable for response, 36 pts (28%) had relapsed (n=33) or refractory (n=3) Burkitt or Burkitt-like leukemia/lymphoma and are the subject of this analysis. Early and late relapse were defined as relapse <6 months and ≥6 months from the time of first remission, respectively. Overall response rate (ORR) defined as the composite of complete remission (CR) and partial remission (PR), relapse-free survival (RFS), and overall survival (OS) were evaluated.
Results: The median age of the evaluable population was 51 years (range, 18-76 years). Among the 36 relapsed/refractory pts, initial presentation was leukemia in 29 pts and lymphoma in 7 pts. Initial treatment was hyper-CVAD alone in 11 pts, hyper-CVAD plus rituximab (R-hyper-CVAD) in 24 pts, and hyper-CVAD plus ofatumumab in 1 pt. Among the 33 relapsed pts, 30 had achieved CR to frontline treatment and 3 had only achieved PR. The median time to first relapse was 6.6 months (range 0.7-75.3 months). Twelve pts (36%) and 21 pts (64%) experienced early and late relapse, respectively.
Thirty relapsed/refractory pts received at least one salvage regimen, 29 of whom were evaluable for response. The backbone salvage regimens received were hyper-CVAD in 13 pts (43%), ICE in 6 (14%), EPOCH in 2 (7%), MOAD in 2 (7%), and miscellaneous regimens in 7 (23%). Subsequent stem cell transplant (SCT) was performed in 6 pts (allogeneic, n=3; autologous, n=3). The ORR to salvage chemotherapy was 38% (CR, n=8; PR, n=3); 18 pts were refractory. Among the 18 pts with late relapse who received salvage therapy, the ORR was 61%. In contrast, of the 11 pts who were refractory to frontline therapy or had early relapse, none responded to salvage therapy (P<0.001 vs. pts with remission duration ≥6 months).
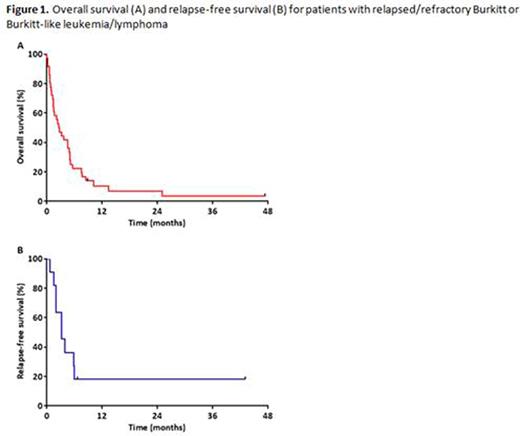
The median OS for the entire cohort (measured from time of relapse or treatment failure) was 2.7 months, with a 1-year OS rate of 10% (Figure 1A). Among responding pts, the median RFS was 3.3 months, with a 1-year RFS rate of 18% (Figure 1B). Only 2 pts were still alive without relapse at last follow-up, one who had undergone salvage autologous SCT and one who had a late relapse 75 months after initial diagnosis and was treated with R-EPOCH without SCT consolidation. Pts who responded to salvage treatment had superior OS compared to those who did not (median OS: 7.5 months vs. 1.5 months, respectively; P<0.001). Pts with late relapse had a median OS of 5.0 months, compared to only 1.4 months for those who were refractory to frontline therapy or experienced early relapse (P<0.001).
Conclusions: Pts with relapsed/refractory Burkitt leukemia/lymphoma have very poor outcomes, with an ORR to salvage therapy of 38% and a median OS of only 2.7 months. Pts with a remission duration after frontline therapy of ≥6 months have superior outcomes, although the OS of this group is still poor. Novel treatment strategies are needed for pts with relapsed/refractory disease.
Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding. Wierda:Abbvie: Research Funding; Acerta: Research Funding; Novartis: Research Funding; Gilead: Research Funding; Genentech: Research Funding. Thompson:Pharmacyclics: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.