Abstract
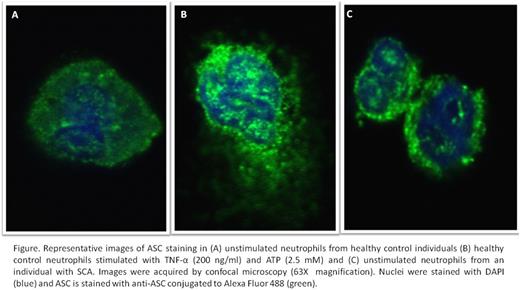
Sickle cell anemia (SCA) is associated with a chronic inflammatory state. The inflammasome complex, responsible for IL-1β and IL-18 cytokine maturation and release, is formed by pattern recognition receptors (PRRs), such as the NLRP3 protein, which recognize damage (or pathogen) associated molecular patterns (DAMPs), in turn recruiting the ASC adapter protein, and cleaving pro-caspase-1, which processes pro-IL-1β and pro-IL-18 into their bioactive forms. While elevated plasma IL-1β and IL-18 levels have been reported in SCA, organization of the inflammasome and its components, and the cells in which this occurs, have yet to be identified in the disease. We aimed to determine whether inflammasome assembly occurs in the leukocytes of individuals with SCA, and associate this formation with levels of some circulating inflammatory DAMPs. Leukocytes (separated by ficoll or percoll gradients) and plasma were obtained from healthy control individuals (CON) and SCA patients in steady state (SCA) not on hydroxyurea therapy. Plasma cytokines and DAMPs were quantified by colorimetric/ bioluminescence assays/ ELISA. Caspase-1 activity was determined in phenotypically-characterized leukocyte populations by flow cytometry (Fam-FlicaTM kit). Both IL-1β and IL-18, hallmarks of inflammasome formation, were significantly increased in SCA plasma, compared to CON (IL-1β: 0.531±0.221, 0.056±0.026 pg/ml; IL-18: 884.0±240.7, 304.8±32.4 pg/ml, for SCA [N=13] and CON [N=11], respectively; P<0.01). Caspase-1 activity was augmented in SCA neutrophils, when compared to CON (2.16±0.36 %, 0.86±0.09 % caspase-1 positivity [N=10/13], respectively; P<0.001), the equivalent of approximately 8.47±1.78 x104 caspase-positive neutrophils/ml in the circulation of these patients. This augmented caspase-1 activity was associated with an elevated secretion of IL-1β from SCA neutrophils, compared to that of CON neutrophils (10.6±1.5 pg/106 cells, 2.1±0.5 pg/106 cells [N=8/9] respectively; P<0.001, during 2h, 37oC). In contrast, although some basal caspase-1 activity was observed in CON CD14+CD16- and CD14+CD16+ monocytes, this activity was not significantly different in SCA monocytes (P>0.05). Accordingly, IL-1β secretion from SCA monocytes was not significantly augmented (P>0.05). Interestingly, while caspase-1 inhibition (co-incubation with 40 µM YVAD-FMK) abrogated IL-1β release from SCA neutrophils (2x106 cell/ml) during 2 h (decreased from 18.9±1.9 pg/ml to 9.8±1.1 pg/ml; N=5, P<0.05), consistent with inflammasome-dependent IL-1β production, NLRP3 inhibition (5µM MCC950 co-incubation) did not affect SCA neutrophil IL-1β release (18.6±3.0 pg/ml; N=5), suggesting that an alternative PPR may participate in the SCA neutrophil inflammasome [MCC 950 inhibitor efficiency was confirmed using a NLRP3 neutrophil inflammasome control]. Furthermore, confocal microscopy and immunofluorescence indicated augmented ASC activity in unstimulated SCA neutrophils, compared to CON neutrophils (Figure 1). We also determined the concentrations of some circulating DAMPs in CON [N≥7] and SCA [N≥11] subjects. Heme, HSP70 and HMGB1 were all significantly (P<0.05) elevated in SCA plasma, compared to CON plasma (Heme: 18.9±2.7, 62.6±7.8 µM; HSP70: 5.53±0.51, 9.41±1.09; HMGB1: 1.6±0.25, 3.6±0.5; for CON and SCA, respectively), while no significant modulations in ATP or IL-33 were observed in SCA plasma (data not shown; P>0.05). However, no significant correlations were observed between DAMP concentrations and plasma IL-1β or neutrophil caspase activity (P>0.05). In conclusion, data indicate that augmented inflammasome assembly occurs in the neutrophils of individuals with SCA; in contrast, we found no evidence of augmented inflammasome activation in the monocytes of these same individuals. Given the high number of neutrophils in the circulation of SCA patients, it seems reasonable to assume that these cells may contribute to augmented IL-1β and IL-18 processing in this disease. DAMPs, associated with both red cell destruction and ischemia-reperfusion injury, were found elevated in the plasma of the SCA individuals studied and may, collectively, contribute to triggering inflammatory pathways, including inflammasome formation. In conclusion, we present data to confirm that inflammasome assembly occurs in sickle cell anemia and may represent a therapeutic target for SCA.
Conran:Bayer AG: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.