Key Points
Low doses of donor iNKT infusion prevent and reverse murine cGVHD.
iNKT efficacy in treating established cGVHD is dependent on donor Treg expansion.
Abstract
Chronic graft-versus-host-disease (cGVHD) can cause multiorgan system disease, typically with autoimmune-like features, resulting in high mortality and morbidity caused by treatment limitations. Invariant natural killer T cells (iNKTs), a small population characterized by expression of a semi-invariant T-cell receptor, rapidly produce copious amounts of diverse cytokines on activation that exert potent immune regulatory function. Here, we show that iNKTs are significantly reduced in a cGVHD murine model that recapitulates several aspects of autoimmunity and organ fibrosis observed in patients with cGVHD. Low iNKT infused doses effectively prevented and, importantly, reversed established cGVHD, as did third-party iNKTs. iNKTs suppressed the autoimmune response by reducing the germinal center (GC) reaction, which was associated with an increase in total Tregs and follicular Tregs (Tfr) that control the GC reaction, along with pathogenic antibody production. Treg depletion during iNKT infusions completely abolished iNKT efficacy in treating cGVHD. iNKT cell interleukin 4 production and GC migration were critical to cGVHD reversal. In vivo stimulation of iNKT cells by α-galactosyl-ceramide was effective in both preventing and treating cGVHD. Together, this study demonstrates iNKT deficiency in cGVHD mice and highlights the key role of iNKTs in regulating cGVHD pathogenesis and as a potentially novel prophylactic and therapeutic option for patients with cGVHD.
Introduction
Chronic graft-versus-host-disease (cGVHD) is the major cause of nonrelapse morbidity and mortality that lacks effective and safe therapies.1 Preclinical models have elucidated autoimmunity as the key of cGVHD pathogenesis, in which dysregulated germinal center (GC) and extrafollicular B-cell responses can lead to the production of autoantibodies that initiate fibrotic responses.2 Although Tregs can treat established murine cGVHD,3 clinical Treg production can be challenging, and sustaining Treg persistence likely requires an interleukin 2 (IL-2) source that is suppressed by Tregs. Thus, we have explored an alternative immune regulatory population, iNKT cells, that are not exquisitely IL-2 sensitive and are highly effective in limited cell numbers in preventing acute GVHD (aGVHD).4-6 iNKT cells have immune regulation function,7 conferred by the rapid production of immune regulatory cytokines such as IL-4 and IL-10,8,9 and promote Treg expansion, perhaps through a myeloid cell intermediate.5,10 iNKT deficiency has been linked to autoimmune diseases (eg, type 1 diabetes, rheumatoid arthritis, and lupus).11-13 Donor graft iNKTs content and their capacity to expand have been associated with GVHD risk.14-16 Total lymphoid irradiation and antithymocyte globulin conditioning in allo-HSCT patients result in an increase in the ratio of host iNKTs to conventional CD4+/CD8+ T cells, which can reduce aGVHD in murine models and patients.17,18 Here, we used a murine model of cGVHD induced by GC reaction that results in antibody production, deposition, and triggering of monocytes/macrophages to cause systemic fibrosis to evaluate the role of iNKT in preventing and treating cGVHD.
Study design
Mice and bone marrow transplantation
C57BL/6 (B6) (Charles River), B10.BR (JAX), CXCR5−/−, and IL-4−/− on B6 background (JAX) and B6.Foxp3.Luci.DTR-4 mice (gift from Professor Günter Hämmerling) were housed in a pathogen-free facility and used with Institutional Animal Care and Use Committee approval. To induce cGVHD, B10.BR mice were given cyclophosphamide and total-body irradiation pretransplant and either B6 bone marrow (BM) only (no cGVHD) or BM with 75 000 purified T cells (cGVHD).2
iNKT isolation and Treg depletion
iNKT cells were fluorescence-activated cell sorter sorted from CD1d-PBS57 tetramer-enriched B6 splenocytes6 to high purity (>95%) and maintained cytokine-producing function (not shown). iNKTs were infused at 50 000 or 100 000 doses at the indicated times. Where stated, Tregs were depleted in B6.Foxp3.Luc-DTR-4 mice by diphtheria toxin (DT; 0.1 μg/mouse) injections before and after iNKTs infusion (days −2, −1, 1 and 2).
cGVHD evaluation
cGVHD was evaluated by pulmonary functional tests (PFTs) and trichrome staining.2,19 Lung hydroxyproline quantification was per manufacturer’s instructions (Sigma MAK008). GC reaction was evaluated by immunofluorescence staining and flow cytometry.2 Bioluminescent imaging was performed using IVIS Spectrum.
Results and discussion
Therapeutic iNKT cell infusion reversed established cGVHD
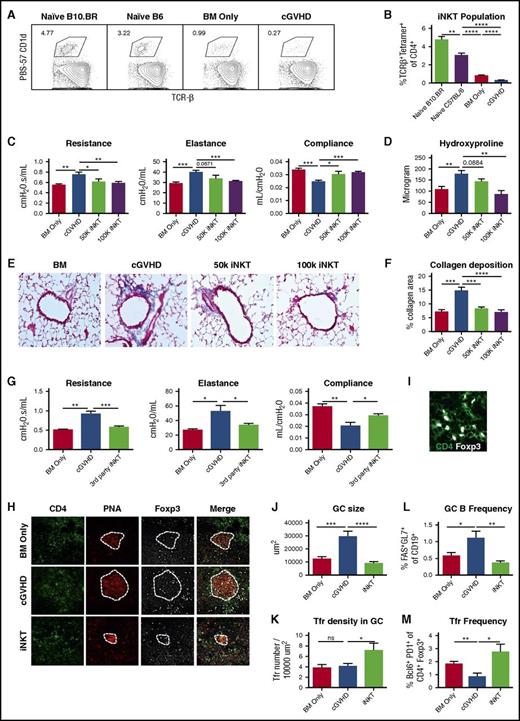
To examine whether the iNKT pool in cGVHD is deficient, we analyzed splenic iNKTs from early-phase cGVHD mice (day 28) and naive donor and host strain mice. cGVHD mice have significantly lower splenic iNKTs than naive mice and BM controls (Figure 1A-B). Thus, we explored adoptive iNKT transfer to treat cGVHD. Donor iNKTs were infused to cGVHD on days 28 and 42 posttransplantation. iNKTs (50 000 or 100 000 cells) reversed lung cGVHD measured by PFTs (Figure 1C). Total lung hydroxyproline (Figure 1D) and collagen deposition around peribronchial and perivascular areas (Figure 1E-F) were reduced (50 000 cells) or completely reversed (100 000 cells; this dose was used for subsequent studies). iNKT cells from a third strain (Balb/c) reversed cGVHD, as well as iNKT from the donor strain (Figure 1G). iNKT infusion reduced GC area (Figure 1H,J) and increased Tfr density (Figure 1I,K). Flow cytometry analysis of day 55 splenocytes confirms that iNKT reduces GC B and increases Tfr frequency (Figure 1L,M). Thus, donor iNKT reversal of established cGVHD was likely a result of increased Tfr frequency.
Therapeutic iNKT cell infusion reversed established cGVHD. B10.BR mice were conditioned by 2 doses of cyclophosphamide (120 mg/kg body weight, intraperitoneally) on day 3 and day 2 of transplantation. On day 1, B10.BR mice were irradiated (830 Gy by X-ray) and then infused with T-cell–depleted BM only or with 75 000 purified T cells to induce cGVHD. (A-B) On day 28 after transplantation, splenocytes were harvested from BM-only mice and cGVHD mice and naive mice of donor and recipient strains. Cells were stained with fluorochrome conjugated PBS57-CD1D tetramer, anti-CD4, anti-TCR-β, and viability dye. iNKT cells were identified by CD4+ TCR-β+PBS57-CD1D+ live cells. (A) Gating of iNKT cells. Cells were gated on live CD4+ T cells. (B) Frequency of iNKT is significantly reduced in cGVHD mice. (C-D) On days 28 and 42 posttransplantation, fluorescence-activated cell sorter sorted CD45.1 B6 iNKTs were infused to some cGVHD mice at a lower (50 000) or higher (100 000) dose. (C) Pulmonary function tests (PFTs), including resistance, elastance, and compliance, were performed on day 56 posttransplantation. iNKT infusion significantly improves the pulmonary function. (D) Hydroxyproline was measured in the lungs of mice from (C). iNKT infusion at the 100 000 cell dose significantly reduces hydroxyproline. (E) Trichrome staining that identifies collagen was performed on cryosections of lungs and imaged at 200×. (F) Collagen deposition was quantified by measuring the blue area by Fiji software. iNTK infusion significantly reduce collagen deposition in the lung. (G) cGVHD was established as previously described. Mice received Balb/c iNKT cells on days 28 and 42. Balb/c iNKT cells reverse cGVHD. (H) Cryosections of spleen (day 56) were stained with fluorochrome conjugated anti-CD4 , anti-Foxp3 (eFluor660), and peanut-agglutinin (PNA) (Rhodamine) and imaged by Olympus FV1000 system at 400×. Dotted lines delineate GC areas by PNA staining. (I) Follicular Tregs were identified as CD4+Foxp3+ cells within the GC areas. (J) Average GC size is decreased by iNKT. (K) Follicular Treg density is increased by iNKT. (L-M) Splenic GC B cells and follicular Tregs frequencies were determined by flow cytometry on day 55 post-transplantation. iNKT infusion decreases GC B and increases follicular Treg frequencies. Unpaired student t-test was used when comparing 2 groups. Data shown are representative of 2 to 4 independent experiments with 5 to 8 mice per group, except (G) with 1 experiment with 5 to 8 mice. *P < .05; **P < .01; ***P < .001; ****P < .0001.
Therapeutic iNKT cell infusion reversed established cGVHD. B10.BR mice were conditioned by 2 doses of cyclophosphamide (120 mg/kg body weight, intraperitoneally) on day 3 and day 2 of transplantation. On day 1, B10.BR mice were irradiated (830 Gy by X-ray) and then infused with T-cell–depleted BM only or with 75 000 purified T cells to induce cGVHD. (A-B) On day 28 after transplantation, splenocytes were harvested from BM-only mice and cGVHD mice and naive mice of donor and recipient strains. Cells were stained with fluorochrome conjugated PBS57-CD1D tetramer, anti-CD4, anti-TCR-β, and viability dye. iNKT cells were identified by CD4+ TCR-β+PBS57-CD1D+ live cells. (A) Gating of iNKT cells. Cells were gated on live CD4+ T cells. (B) Frequency of iNKT is significantly reduced in cGVHD mice. (C-D) On days 28 and 42 posttransplantation, fluorescence-activated cell sorter sorted CD45.1 B6 iNKTs were infused to some cGVHD mice at a lower (50 000) or higher (100 000) dose. (C) Pulmonary function tests (PFTs), including resistance, elastance, and compliance, were performed on day 56 posttransplantation. iNKT infusion significantly improves the pulmonary function. (D) Hydroxyproline was measured in the lungs of mice from (C). iNKT infusion at the 100 000 cell dose significantly reduces hydroxyproline. (E) Trichrome staining that identifies collagen was performed on cryosections of lungs and imaged at 200×. (F) Collagen deposition was quantified by measuring the blue area by Fiji software. iNTK infusion significantly reduce collagen deposition in the lung. (G) cGVHD was established as previously described. Mice received Balb/c iNKT cells on days 28 and 42. Balb/c iNKT cells reverse cGVHD. (H) Cryosections of spleen (day 56) were stained with fluorochrome conjugated anti-CD4 , anti-Foxp3 (eFluor660), and peanut-agglutinin (PNA) (Rhodamine) and imaged by Olympus FV1000 system at 400×. Dotted lines delineate GC areas by PNA staining. (I) Follicular Tregs were identified as CD4+Foxp3+ cells within the GC areas. (J) Average GC size is decreased by iNKT. (K) Follicular Treg density is increased by iNKT. (L-M) Splenic GC B cells and follicular Tregs frequencies were determined by flow cytometry on day 55 post-transplantation. iNKT infusion decreases GC B and increases follicular Treg frequencies. Unpaired student t-test was used when comparing 2 groups. Data shown are representative of 2 to 4 independent experiments with 5 to 8 mice per group, except (G) with 1 experiment with 5 to 8 mice. *P < .05; **P < .01; ***P < .001; ****P < .0001.
iNKT reversed cGVHD through donor Treg expansion
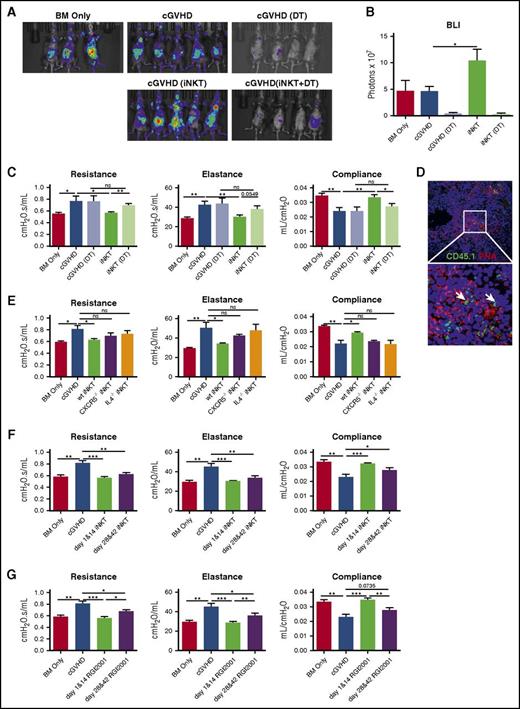
As a result of the reduced Treg frequency observed in both patients with cGVHD20 and our murine model,3 and reversal of cGVHD in murine model with Treg infusion,3 we examined whether iNKT reversed cGVHD through Treg expansion. Donor B6.Foxp3.Luci.DTR-4 mice21 permitted both tracking expansion and eliminating Foxp3 expressing Tregs; iNKT increased Foxp3 signal intensity by 2-fold (Figure 2A-B). Peri-infusion donor Treg depletion completely abrogated iNKT-mediated protection, as indicated by PFTs (Figure 2C), confirming a Treg-dependent mechanism.
iNKT reversed cGVHD through donor Treg expansion and prevented the onset of cGVHD. (A-C) cGVHD was established as per Figure 1, except that BM and T cells were harvested from B6.Foxp3.Luci.DTR-4 mice. Group 1 and 2 are BM only and cGVHD control, as described before. Groups 3 through 5 are cGVHD mice that received DT (group 3), iNKT infusion on day 28 and day 42 (group 4), or iNKT infusion and DT (group 5). (A) On day 43, mice were imaged by the Spectrum In Vivo Imaging system. (B) Quantification of the bioluminescent imaging signal shows depletion of Tregs by DT and expansion of Tregs by iNKT infusion. (C) PFTs were assessed as described in Figure 1. Treg depletion by DT injection completely abolishes iNKTs efficacy. (D) iNKT cells (white arrow) were identified by CD45.1+ in the GC area. (E) Mice were transplanted as per Figure 1. iNKT cells from wild-type, CXCR5−/−, or IL-4−/− mice were infused to transplanted mice on days 28 and 42. iNKT cells from CXCR5−/− or IL-4−/− mice lost the ability to reverse cGVHD. (F) cGVHD mice were infused with B6 iNKTs either on days 1 and 14 (prophylaxis) or on days 28 and 42 (therapy). Prophylactic iNKT infusion completely blocks cGVHD. (G) Prophylactic or therapeutic RGI2001 (2.5 μg/mouse) was given to transplanted mice. PFTs suggest RGI2001 prevents and reverses cGVHD. Five to 8 mice per group were analyzed for each assay. Significance: *P < .05; **P < .01; ***P < .001; ****P < .0001.
iNKT reversed cGVHD through donor Treg expansion and prevented the onset of cGVHD. (A-C) cGVHD was established as per Figure 1, except that BM and T cells were harvested from B6.Foxp3.Luci.DTR-4 mice. Group 1 and 2 are BM only and cGVHD control, as described before. Groups 3 through 5 are cGVHD mice that received DT (group 3), iNKT infusion on day 28 and day 42 (group 4), or iNKT infusion and DT (group 5). (A) On day 43, mice were imaged by the Spectrum In Vivo Imaging system. (B) Quantification of the bioluminescent imaging signal shows depletion of Tregs by DT and expansion of Tregs by iNKT infusion. (C) PFTs were assessed as described in Figure 1. Treg depletion by DT injection completely abolishes iNKTs efficacy. (D) iNKT cells (white arrow) were identified by CD45.1+ in the GC area. (E) Mice were transplanted as per Figure 1. iNKT cells from wild-type, CXCR5−/−, or IL-4−/− mice were infused to transplanted mice on days 28 and 42. iNKT cells from CXCR5−/− or IL-4−/− mice lost the ability to reverse cGVHD. (F) cGVHD mice were infused with B6 iNKTs either on days 1 and 14 (prophylaxis) or on days 28 and 42 (therapy). Prophylactic iNKT infusion completely blocks cGVHD. (G) Prophylactic or therapeutic RGI2001 (2.5 μg/mouse) was given to transplanted mice. PFTs suggest RGI2001 prevents and reverses cGVHD. Five to 8 mice per group were analyzed for each assay. Significance: *P < .05; **P < .01; ***P < .001; ****P < .0001.
Infused CD45.1 iNKT cells were detected in GC areas 5 days after infusion (Figure 2D). To test whether GC migration and IL-4 production are required for iNKT’s protective function in cGVHD, iNKTs from CXCR5−/− or IL-4−/− mice were infused. Neither CXCR5−/− nor IL-4−/− iNKTs were able to reverse cGVHD (Figure 2E). Taken together with the high Tfr frequency conferred by iNKT infusion, these data point to in situ Tfr expansion in GC area by iNKT through an IL-4 dependent mechanism as a key mechanistic underpinning of iNKT-mediated cGVHD therapy.
Pharmacologic activation of iNKT is effective in preventing and reversing cGVHD
To determine the potential of iNKT in preventing cGVHD, donor iNKTs were given on days 1 and 14 (prophylaxis) or on days 28 and 42 (therapy). PFTs showed that prophylactic infusion completely blocked cGVHD, resulting in modestly more robust protection compared with therapeutic infusion (Figure 2F). Prophylactic iNKT efficacy may be advantageous because of the expansion of host radio-resistant Tregs or donor Tregs in the graft that suppress inflammation and tissue damage, preventing cGVHD initiation, as well as easier disease prevention than reversal. In support of this latter hypothesis, and further demonstrating the potential iNKT therapeutic benefits, we demonstrated the efficacy of RGI2001, a liposomal formulation of a-galactosylceramide, in both treating and, to an even greater extent, preventing cGVHD (Figure 2G).
Because aGVHD is a critical risk factor for cGVHD,1 managing aGVHD can significantly reduce cGVHD incidence. iNKT infusion (25 000-100 000 cells) protected mice from aGVHD in a dose-dependent manner through Treg expansion.5 These data and ours suggest iNKT cell infusion protects from both aGVHD and cGVHD, which we speculate is because both diseases are associated with an inadequate Treg pool, and hence T effector/Treg ratio.20,22 The fact that iNKT infusion is useful for both aGVHD and cGVHD offers the possibility for optimal treatment of patients with dual aGVHD and cGVHD components.23
Compared with Treg infusion, iNKT infusion required fewer cells (100 000 iNKT vs 500 000 Treg in the same model) to reach optimal effect.3,24 In addition, iNKT cells have inherent antiviral and antitumor abilities25 that are desirable for patients with cGVHD. iNKT cells are persistent in host, as they can be detected in spleen, liver, and lung at least 2 weeks after infusion (not shown). A detailed migration/expansion profile of iNKT cells in cGVHD mice is to be studied. In summary, this study provides evidence that iNKT infusion and expansion are promising prophylactic and therapeutic options for patients with cGVHD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Immunofluorescence images and bioluminescent imaging were acquired using the Olympus FV1000 upright confocal and IVIS Spectrum In Vivo imaging system at the University of Minnesota-University Imaging Centers (http://uic.umn.edu).
The following funding sources contributed to this work: National Institutes of Health, National Cancer Institute grants P01 CA142106-06A1, 5P01-CA047741-20, P01-CA049605; National Institutes of Health, National Heart, Lung, and Blood Institute grants P01 HL075462, R01 HL126530, and K08HL107756; National Institutes of Health, National Institute of Allergy and Infectious Diseases grants P01 AI 056299 and T32 AI 007313; and Leukemia and Lymphoma Society translational research grants 6458-15 and 6462-15.
Authorship
Contribution: J.D. designed the experiments and wrote the paper; J.D., K.P., G.T., D.S., J.B., R.F., O.D., and C.F. performed experiments, discussed results, and edited the paper; A.P.-M. performed histologic analyses; and R.S.N. and B.R.B. contributed to experimental design, discussed results, and edited the paper.
Conflict-of-interest disclosure: The authors declare competing financial interests as a pending patent on iNKT cell infusion to treat cGVHD.
Correspondence: Robert S. Negrin, Center for Clinical Sciences Research Building, Room 2205; 269 Campus Dr, Stanford, CA 94305; e-mail: negrs@stanford.edu; or Bruce R. Blazar, MMC 109, University of Minnesota, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: blaza001@umn.edu.
References
Author notes
R.S.N. and B.R.B. contributed equally to this study.