Key Points
CCL2 is a KIT D816V–induced cytokine targeting microenvironmental cells in mastocytosis in vitro and in vivo.
Serum levels of CCL2 in patients with mastocytosis correlate with advanced disease and poor survival.
Abstract
Systemic mastocytosis (SM) is characterized by abnormal accumulation of neoplastic mast cells harboring the activating KIT mutation D816V in the bone marrow and other internal organs. As found in other myeloproliferative neoplasms, increased production of profibrogenic and angiogenic cytokines and related alterations of the bone marrow microenvironment are commonly found in SM. However, little is known about mechanisms and effector molecules triggering fibrosis and angiogenesis in SM. Here we show that KIT D816V promotes expression of the proangiogenic cytokine CCL2 in neoplastic mast cells. Correspondingly, the KIT-targeting drug midostaurin and RNA interference–mediated knockdown of KIT reduced expression of CCL2. We also found that nuclear factor κB contributes to KIT-dependent upregulation of CCL2 in mast cells. In addition, CCL2 secreted by KIT D816V+ mast cells was found to promote the migration of human endothelial cells in vitro. Furthermore, knockdown of CCL2 in neoplastic mast cells resulted in reduced microvessel density and reduced tumor growth in vivo compared with CCL2–expressing cells. Finally, we measured CCL2 serum concentrations in patients with SM and found that CCL2 levels were significantly increased in mastocytosis patients compared with controls. CCL2 serum levels were higher in patients with advanced SM and were found to correlate with poor survival. In summary, we have identified CCL2 as a novel KIT D816V–dependent key regulator of vascular cell migration and angiogenesis in SM. CCL2 expression correlates with disease severity and prognosis. Whether CCL2 may serve as a therapeutic target in advanced SM remains to be determined in forthcoming studies.
Introduction
Myeloproliferative neoplasms (MPNs) are clonal hematopoietic stem cell diseases characterized by an accumulation of mature and immature blood cells and aberrant tyrosine kinase signaling.1 Increased production of cytokines has been implicated in disease progression and correlates with the symptom burden of patients with classical JAK2 V617F+ MPNs.2-4 Furthermore, marked bone marrow (BM) fibrosis and increased microvessel density in MPN patients have been associated with aberrant cytokine expression and disease progression.5,6 In addition, reduction of cytokine levels is a major effect of JAK2-targeting tyrosine kinase inhibitor treatment in classical MPNs.7 In contrast to the comprehensive data on cytokine expression in classical MPNs, only a few studies addressing the role of cytokines in systemic mastocytosis (SM) have been published.8
SM is a clonal disease of hematopoietic stem cells characterized by mast cell (MC) infiltration in the BM and/or other organs.9 In addition, BM microenvironment alterations, including increased angiogenesis, thickened bone trabeculae, fibrosis, and lymphocytic and eosinophilic infiltrations, are typically found in SM.10 Most patients present with the somatic KIT mutation D816V.11,12 The D816V mutant exhibits constitutively active signaling and induces the recruitment of several downstream pathways, including signal transducer and activator of transcription 5 (STAT5), phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT), the mitogen-activated protein kinase (MAPK), and the mammalian target of rapamycin (mTOR) pathways.13-15 Mutant KIT is considered to be the major driver of pathogenesis in SM. In line with this notion, tyrosine kinase inhibitors targeting KIT D816V showed efficacy in the treatment of advanced SM.9,16,17 The clinical manifestation of the disease is highly variable, ranging from an indolent course to highly aggressive variants with short survival. The World Health Organization (WHO) classification defines 4 major subtypes of SM that are based on clinical findings: indolent systemic mastocytosis (ISM), mastocytosis with an associated hematologic neoplasm (SM-AHN), aggressive systemic mastocytosis (ASM), and mast cell leukemia (MCL).18-21 On the basis of clearly reduced survival and poor outcome compared with ISM, the variants SM-AHN, ASM, and MCL are collectively referred to as advanced SM.22,23
In contrast to classical MPNs, only a limited number of cytokines have been studied in SM. Interleukin-6 (IL-6) was shown to be increased in SM, and plasma levels of IL-6 correlate with disease severity and progression.24-28 In particular, IL-6 was described to be increased in the plasma of SM patients and to correlate with serum tryptase levels, severity of symptoms, disease category, severity of BM pathology, organomegaly, the presence of osteoporosis, and the extent of skin involvement.24-27 A recent study confirmed the prognostic significance of IL-6 in SM.28 High levels of IL-6 were associated with disease severity and also with disease progression in patients with ISM.28 IL-31 has been implicated in the induction of chronic skin inflammation and was also found to be increased in patients with SM.29 We and others have shown that vascular endothelial growth factor and oncostatin M might contribute to changes of the BM microenvironment in SM.30,31 However, other cytokines potentially triggering angiogenesis and fibrosis, which are involved in the complex interplay between neoplastic MC and stromal cells in the BM of patients with SM have not yet been studied.8
The chemokine (C-C motif) ligand 2 (CCL2), also referred to as monocyte chemotactic protein 1 (MCP-1), is a pleiotropic cytokine that recruits inflammatory cells to the sites of inflammation.32 CCL2 primarily acts via the chemokine (C-C motif) receptor 2 (CCR2) that is expressed on monocytes and macrophages, memory T lymphocytes, natural killer cells, and also on various mesenchymal cells.33-35 In particular, CCL2 has been shown to enhance angiogenesis and fibrosis under physiologic conditions as well as in inflammation and cancer.35,36 Various solid tumors express CCL2 that in turn promotes tumor growth and metastasis mainly via its paracrine effect on the tumor microenvironment.37 Consequently, targeting of CCL2 showed promising results in cancer models.35,38-41 By contrast, only limited data are available regarding CCL2 in MPNs. Increased CCL2 serum levels have been described in patients with JAK2 V617F+ MPNs,2,4,42 and MCs were also found to express CCL2.43,44 However, CCL2 has not been studied in patients with SM, and targeting of CCL2 has not been addressed in MPNs.
In this study, we identified CCL2 as a KIT D816V–induced cytokine targeting microenvironmental cells in vitro and in vivo. Furthermore, high serum levels of CCL2 were found in patients with SM, and they correlate with advanced disease and poor survival.
Materials and methods
Reagents
Cell culture media and fetal calf serum (FCS) were obtained from Life Technologies (Carlsbad, CA). Recombinant human stem cell factor (SCF) and granulocyte-macrophage colony-stimulating factor were purchased from PeproTech (Rocky Hill, NJ), IL-6 from Miltenyi Biotec (Auburn, CA), and CCL2 from R&D Systems (Minneapolis, MN). Midostaurin and the PI3K/mTOR inhibitor BEZ235 were kindly provided by Dr E. Buchdunger and Dr P.W. Manley (Novartis Pharma AG, Basel, Switzerland). The MEK (defined as MAPK/extracellular signal-regulated kinase [ERK]) inhibitor PD98059 and the PI3K inhibitor LY294002 were purchased from Calbiochem (La Jolla, CA), and the broadly acting STAT inhibitor piceatannol and the inhibitor of κB (IκB) kinase inhibitor TPCA-1 from Tocris Bioscience (Bristol, United Kingdom).
Patients
The study was conducted in accordance with the Declaration of Helsinki and approved by the local institutional review board (EK#2010/566, EK#2011/404, EK#2014/1018, and EK#2014/1184). Forty-eight patients with mastocytosis were examined (Table 1). Mastocytosis was diagnosed according to WHO criteria.18,19 Routine staging included serum tryptase measurement, BM histology, cytologic examination of BM cells, flow cytometry (CD2 and CD25 on BM MCs), and analysis for KIT D816V by melting point analysis after clamp polymerase chain reaction (PCR) and/or quantitative allele-specific PCR.45,46 Microvessel density was determined by immunohistochemical CD34 staining and counting of all vessels within an examination area of 0.25 mm2. Fibrosis was determined by reticulin staining according to the European consensus system (scale of 0 to 3).47 Serum samples from age- and sex-matched healthy donors were used as a control cohort. Informed consent was obtained in each case.
Cells and cell culture
TF-1 and Mo7e were cultured in RPMI 1640 medium with 10% FCS and granulocyte-macrophage colony-stimulating factor (5 ng/mL), and HMC-1 cells (KIT D816V+ HMC-1.2 clone)48 were cultured in Iscove modified Dulbecco medium with 10% FCS. CD34+ progenitor cells were isolated from cord blood by magnetic-activated cell sorting (Miltenyi Biotec) and cultured in the presence of SCF and IL-6 as described.49 Human umbilical vein endothelial cells (HUVECs) were isolated from umbilical cords according to published techniques and cultured in plates coated with fibronectin (31.25 μg/mL) in M199 medium with 20% FCS and supplements.50
Expression of KIT D816V
The coding sequences of wild-type KIT and KIT D816V were cloned into the lentiviral pWPI vector (kindly provided by Didier Trono, Department of Microbiology and Molecular Medicine, University of Geneva, Geneva, Switzerland). Recombinant lentiviruses were produced as described.31 TF-1 and Mo7e cells were transduced with wild-type KIT, KIT D816V, or the empty vector and sorted for green fluorescent protein (FACSAria; Becton Dickinson, San Jose, CA).
Knockdown experiments
For knockdown of KIT, a lentiviral vector pLKO.2 encoding a short hairpin RNA targeting KIT was used.31 For knockdown of CCL2 or nuclear factor κB1 (NF-κB1), hairpins in a microRNA-E (miR-E) backbone were cloned into the pRRL-based SGEP vector as described.51 Guide sequences of the hairpins are provided in supplemental Table 1, available on the Blood Web site. Production of recombinant vesicular stomatitis virus G glycoprotein pseudotyped lentiviruses and transduction of target cells was performed as described.52
Real-time PCR
Real-time PCR for the initial cytokine screen was performed as described.52 Results were expressed as ΔCt values (ΔCt = CtABL − Ctcytokine). In addition, CCL2 messenger RNA (mRNA) copy numbers were absolutely quantified on a Biorad Real-Time PCR System (Biorad, Foster City, CA) using iTaq SYBR Green Supermix with ROX (Biorad, Hercules, CA) and plasmid standards. CCL2 copy numbers were normalized to Abelson murine leukemia viral oncogene homolog 1 (ABL1) copy numbers and expressed as percent of ABL.53 Primer sequences are provided in supplemental Table 2.
ELISA
To determine the levels of CCL2 protein in cell culture supernatants, an enzyme-linked immunosorbent assay (ELISA; R&D Systems) was used. The lower limit of detection was 50 pg/mL. Patients’ sera were analyzed by a commercial precoated ELISA (R&D Systems) according to the recommendations of the manufacturer. The lower limit of detection was 31.3 pg/mL.
Immunoblotting
Whole-cell extracts were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis, and immunoblotting was carried out by using antibodies against KIT, phospho-KIT, and β-actin as described.54 Cytoplasmatic and nuclear extracts were isolated by using NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Scientific, Waltham, MA) and probed with antibodies against NF-κB, IκB, phospho-IκB, glyceraldehyde-3-phosphate dehydrogenase, and histone H3. A description of antibodies used in this study is provided in supplemental Table 3.
Flow cytometry and cell sorting
Surface expression of CCR2 was determined by using a brilliant violet 421-conjugated CCR2 antibody (clone K036C2, BioLegend) or an isotype matched control antibody. Cells were analyzed by flow cytometry on a FACSCanto II (Becton Dickinson) with FlowJo software (Tree Star, Ashland, OR).49 CD117++ primary MCs were enriched from BM aspirates by fluorescence-activated cell sorting.
Immunohistochemistry
Immunohistochemistry was performed on paraffin-embedded, formalin-fixed BM sections with a Ventana Benchmark Ultra stainer (Ventana, Tucson, AZ) with extended heat-induced epitope retrieval with CC1 buffer and the ultraView Universal DAB Detection Kit (Ventana). Xenotransplant tumor sections were stained with hematoxylin and eosin, chromotrope-aniline blue (CAB), or by immunohistochemistry for Ki-67 or von Willebrand factor. Fibrosis and inflammation in the tumors was graded from 0 to 4. A description of antibodies used in this study is provided in supplemental Table 3.
Boyden chamber and wound-healing assay
Polycarbonate membranes (8 μM pore size, 0.7 cm2 growth area, Corning Incorporated, Corning, NY) were coated with fibronectin (31.25 μg/mL). The lower chambers were filled with medium with or without supernatant from HMC-1 cells. In selected experiments, conditioned medium was pre-incubated with anti-CCL2 antibody (mouse immunoglobulin G1, clone 24822, R&D Systems) or an isotype control antibody for 1 hour at 37°C. HUVECs (2 × 104) were loaded into the top chamber of each well and incubated for 4 hours. Cells attached to the lower side of the membrane were fixed on ice with methanol, washed with phosphate-buffered saline, and stained with 4′,6-diamidino-2-phenylindole. The number of migrated cells was evaluated by fluorescence microscopy (Axio Imager A1 microscope; Zeiss, Oberkochen, Germany).
HUVECs were grown to confluence in 24-well plates, and a scratch was introduced to the monolayer. Medium with or without supernatant from HMC-1 cells was added to the cell layer. In selected experiments, conditioned medium was pre-incubated with anti-CCL2 antibody as described above. Cell layers were examined after 24 hours of incubation with an Axiovert 25 inverted microscope (Zeiss). Migrated cells were counted by using ImageJ software.55 Results were expressed as percent of control.
Animal experiments
Animal studies were approved by the local institutional review committee for animal research (approval No. GZ 68.205/0218-II/3b/2012). In brief, NOD-SCID IL-2Rgamma-null (NSG) mice (The Jackson Laboratory, Bar Harbor, ME) were subcutaneously injected with HMC-1 cells (1 × 107) after lentiviral knockdown of CCL2 (n = 6) or transduction with a nontargeting control vector (n = 5). Each mouse received 2 independent injections into each lower flank. After 3 weeks, tumor nodules were palpable, and tumor size was measured every other day with a slide caliper and calculated by using the formula a2 × b/2 where a is the tumor length and b is the tumor width. After 34 days, mice were euthanized and subcutaneous tumors were weighed, formalin fixed, and paraffin embedded.
Statistical analysis
Data were analyzed by using GraphPad Prism software (GraphPad Software, La Jolla, CA). To compare differences, Student t test (for 2 groups) or one-way analysis of variance (followed by Bonferroni-adjusted post hoc analysis for more than 2 groups) was applied for parametric data, and the Mann-Whitney U test (for 2 groups) or the Kruskal-Wallis test (followed by Dunn’s post hoc analysis for more than 2 groups) was applied for nonparametric data. The survival was estimated by the Kaplan-Meier method and the log-rank test. Results were considered significantly different when the P value was <.05. The reference range for CCL2 was calculated from the healthy control cohort as mean ± 2 standard deviations (SD).
Results
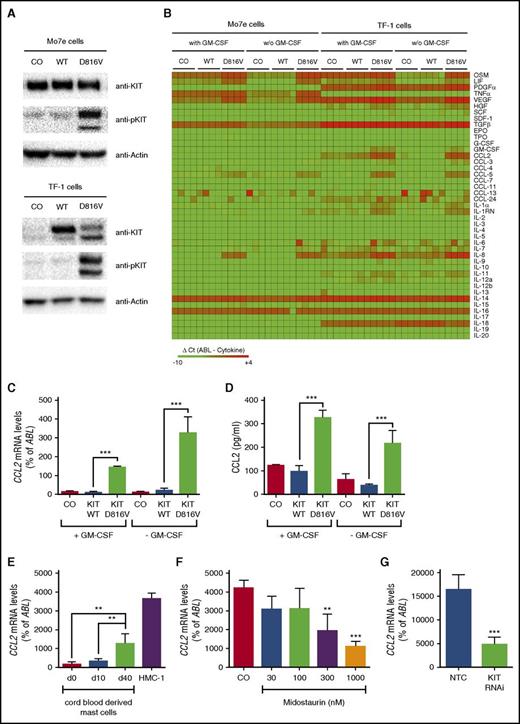
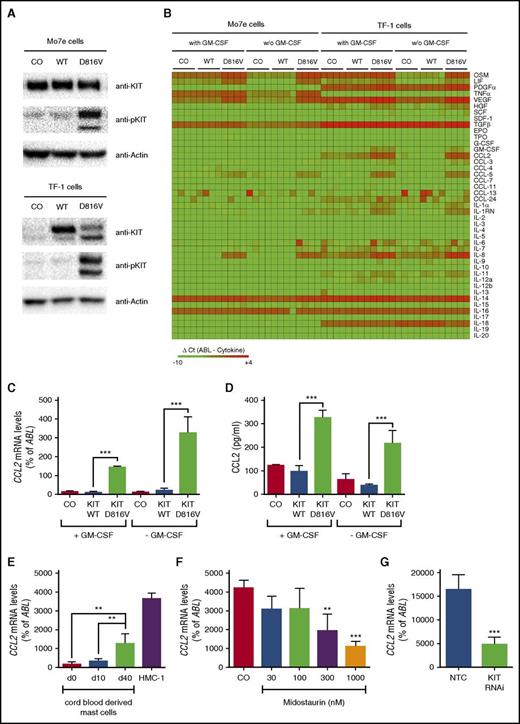
KIT D816V induces expression of CCL2
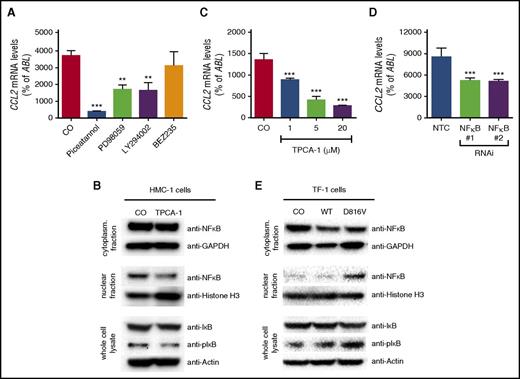
To screen for KIT D816V–dependent expression of cytokines potentially involved in the pathogenesis of SM, expression levels of 43 cytokines were determined in 2 growth factor–dependent human cell lines, Mo7e and TF-1, after lentiviral transduction of KIT D816V (Figure 1A). KIT D816V induced expression of OSM, LIF, IL-8, TNF-α, VEGF, CCL2, and CCL5 in at least 1 cell line model (Figure 1B). In this study, we addressed the role of CCL2 in the pathogenesis of SM. KIT D816V significantly induced CCL2 mRNA expression (Figure 1C) and CCL2 protein secretion (Figure 1D). In line with this finding, CCL2 mRNA levels increased substantially during SCF-mediated differentiation of cord blood–derived MCs (Figure 1E). Moreover, the highest CCL2 levels were observed in the human neoplastic MC line HMC-1 harboring the KIT D816V mutation (Figure 1E). To confirm that CCL2 expression in neoplastic MCs is dependent on KIT D816V, HMC-1 cells were treated with midostaurin, a multikinase inhibitor reportedly suppressing the kinase activity of mutant KIT. Midostaurin was found to reduce expression of CCL2 in HMC-1 cells in a dose-dependent manner (Figure 1F). Similar effects were observed after RNA interference (RNAi) –mediated knockdown of KIT in HMC-1 cells (Figure 1G). Together, these data show that KIT D816V promotes expression of CCL2.
KIT D816V induces expression of CCL2. (A) Mo7e and TF-1 cells were transduced with the empty vector (CO), wild-type (WT) KIT, or KIT D816V. Then, expression of total and phosphorylated KIT (pKIT) were determined by immunoblotting. Actin served as a loading control. (B) Mo7e and TF-1 cells were transduced as described and cultured in the absence or presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) for 16 hours. Green fluorescent protein (GFP) –positive cells were purified by fluorescence-activated cell sorting (FACS), and expression of cytokines was determined by quantitative real-time PCR. Results are expressed as ΔCt values (ΔCt = CtABL − Ctcytokine). Samples without detectable mRNA expression were set to a ΔCt value of −10.0. Data from 4 experiments are shown. (C,D) TF-1 cells were transduced and cultured as described above. Expression of CCL2 was determined by (C) real-time PCR of cells or (D) ELISA of cell culture supernatants. (E) Cord blood–derived MCs harvested at different time points and KIT D816V+ HMC-1 cells were analyzed for expression of CCL2. (F) HMC-1 cells were treated with midostaurin for 16 hours, and expression of CCL2 was determined by real-time PCR. (G) Expression of CCL2 was analyzed in HMC-1 cells after transduction with a short hairpin RNA–targeting KIT (KIT RNAi) or a nontargeting control (NTC). Results represent the mean ± standard deviation (SD) of at least 3 independent experiments. **P < .01, ***P < .001.
KIT D816V induces expression of CCL2. (A) Mo7e and TF-1 cells were transduced with the empty vector (CO), wild-type (WT) KIT, or KIT D816V. Then, expression of total and phosphorylated KIT (pKIT) were determined by immunoblotting. Actin served as a loading control. (B) Mo7e and TF-1 cells were transduced as described and cultured in the absence or presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) for 16 hours. Green fluorescent protein (GFP) –positive cells were purified by fluorescence-activated cell sorting (FACS), and expression of cytokines was determined by quantitative real-time PCR. Results are expressed as ΔCt values (ΔCt = CtABL − Ctcytokine). Samples without detectable mRNA expression were set to a ΔCt value of −10.0. Data from 4 experiments are shown. (C,D) TF-1 cells were transduced and cultured as described above. Expression of CCL2 was determined by (C) real-time PCR of cells or (D) ELISA of cell culture supernatants. (E) Cord blood–derived MCs harvested at different time points and KIT D816V+ HMC-1 cells were analyzed for expression of CCL2. (F) HMC-1 cells were treated with midostaurin for 16 hours, and expression of CCL2 was determined by real-time PCR. (G) Expression of CCL2 was analyzed in HMC-1 cells after transduction with a short hairpin RNA–targeting KIT (KIT RNAi) or a nontargeting control (NTC). Results represent the mean ± standard deviation (SD) of at least 3 independent experiments. **P < .01, ***P < .001.
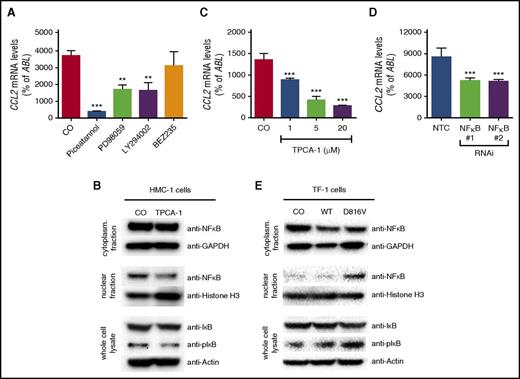
Role of NF-κB signaling in KIT D816V–dependent CCL2 expression
To assess the role of signaling pathways downstream of KIT D816V on CCL2 expression, pharmacologic inhibitors targeting key signaling molecules known to be activated by KIT D816V, namely MAPK, PI3K, and STAT5, were applied. Piceatannol, a compound directed against multiple targets, including STAT5, the MAPK inhibitor PD98059, and the rather unspecific PI3K inhibitor LY294002 reduced expression of CCL2 in HMC-1 cells (Figure 2A). In contrast, the more specific PI3K inhibitor BEZ235 showed no effect (Figure 2A). This suggests that LY294002 does not inhibit CCL2 expression via the PI3K pathway. Piceatannol and LY294002 have also been shown to affect NF-κB signaling.56,57 Therefore, a specific NF-κB inhibitor, TPCA-1, was used to analyze the dependency of CCL2 expression on NF-κB in HMC-1 cells. Indeed, treatment of HMC-1 cells with TPCA-1 reduced the nuclear fraction of NF-κB and also the phosphorylation of the NF-κB inhibitor IκB (Figure 2B) and resulted in a dose-dependent reduction of CCL2 expression (Figure 2C). In addition, we silenced expression of NF-κB1 in neoplastic MCs by RNAi. Knockdown of NF-κB1 significantly reduced expression of CCL2, confirming the specificity of the effect (Figure 2D). Complementary to this observation, lentiviral expression of KIT D816V resulted in activation of the NF-κB pathway in TF-1 cells, as demonstrated by nuclear translocation of NF-κB and phosphorylation of IκB (Figure 2E). Together, our data show that KIT D816V promotes expression of CCL2 through activation of NF-κB in neoplastic MCs.
Role of NF-κB in KIT D816V–dependent expression of CCL2. (A) Effects of piceatannol (200 µM), PD98059 (50 µM), LY294002 (20 µM), or BEZ253 (1 µM) on expression of CCL2 in HMC-1 cells. (B) HMC-1 cells were treated with TPCA-1 (20 µM), and the effect on NF-κB signaling was determined by immunoblotting with antibodies against NF-κB in cytoplasmic and nuclear fractions as well as IκB and phosphorylated IκB (pIκB) in whole-cell lysates. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), histone H3, and β-actin served as the respective loading controls. (C-D) Effect of TPCA-1 treatment (C) as indicated or (D) of RNAi-mediated knockdown of NF-κB1 compared with an NTC on expression of CCL2 in HMC-1 cells. (E) TF-1 cells were transduced with CO, WT KIT, or KIT D816V, and NF-κB signaling was analyzed as described above. Results represent the mean ± SD of at least 3 independent experiments. **P < .01, ***P < .001.
Role of NF-κB in KIT D816V–dependent expression of CCL2. (A) Effects of piceatannol (200 µM), PD98059 (50 µM), LY294002 (20 µM), or BEZ253 (1 µM) on expression of CCL2 in HMC-1 cells. (B) HMC-1 cells were treated with TPCA-1 (20 µM), and the effect on NF-κB signaling was determined by immunoblotting with antibodies against NF-κB in cytoplasmic and nuclear fractions as well as IκB and phosphorylated IκB (pIκB) in whole-cell lysates. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), histone H3, and β-actin served as the respective loading controls. (C-D) Effect of TPCA-1 treatment (C) as indicated or (D) of RNAi-mediated knockdown of NF-κB1 compared with an NTC on expression of CCL2 in HMC-1 cells. (E) TF-1 cells were transduced with CO, WT KIT, or KIT D816V, and NF-κB signaling was analyzed as described above. Results represent the mean ± SD of at least 3 independent experiments. **P < .01, ***P < .001.
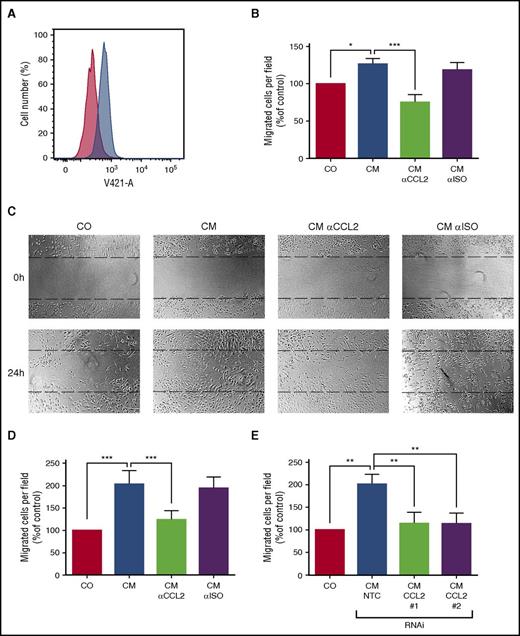
MC-derived CCL2 promotes endothelial cell migration in vitro
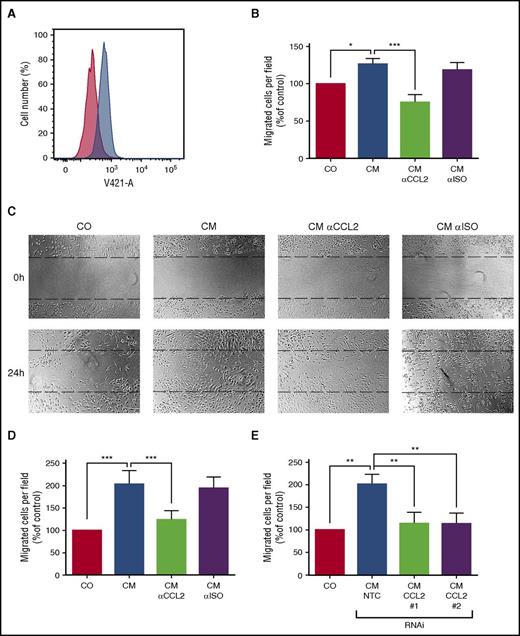
CCL2 has been reported to promote angiogenesis and thus may be important for the BM alterations associated with SM. Therefore, we analyzed the effect of conditioned medium from HMC-1 cells on HUVECs. As expected, HUVECs were found to express the CCL2 receptor CCR2 (Figure 3A) and to migrate against recombinant CCL2 in a modified Boyden chamber assay. Conditioned medium from neoplastic MCs also induced migration of HUVECs, and pre-incubation with a neutralizing antibody against CCL2 significantly reduced their migratory response (Figure 3B). Furthermore, the supernatant of KIT D816V+ HMC-1 cells enhanced migration of HUVECs in a wound-healing assay. Again, a neutralizing antibody against CCL2 abolished this effect (Figure 3C-D). In addition, we silenced expression of CCL2 in neoplastic MCs by RNAi to further confirm specificity. Knockdown of CCL2 reduced the effect of conditioned medium of neoplastic MCs to the baseline level in the wound-healing assay (Figure 3E; supplemental Figure 1). Together, these data suggest that CCL2 might contribute to BM microenvironment alterations in SM patients.
CCL2 promotes chemotaxis and migration of endothelial cells in vitro. (A) HUVECs were stained with an anti-CCR2 antibody (blue histogram) or isotype control (red histogram) and analyzed by FACS. (B) Migration of HUVECs toward conditioned medium of neoplastic MC (CM) was determined using a modified Boyden chamber assay. CM was pre-incubated with an antibody against CCL2 or an isotype control antibody (αISO) to determine the specificity of the observed effect. The number of migrated cells per field was counted and normalized to control level. (C-E) Migration of HUVECs in a scratch assay. The upper panel of (C) shows the scratch before and the lower panel after 24 hours of incubation with CM. CM was pre-incubated with an antibody against CCL2 or an isotype control antibody (αISO) as described above. The number of cells in the scratch was counted and normalized to control level. (D) Respective numbers of mean ± SD of at least 3 independent experiments. (E) In similar experiments, conditioned medium from neoplastic MCs after transduction with 2 different short hairpin RNAs (shRNAs) targeting CCL2 (#1 and #2) or an NTC was used. Results represent the mean ± SD of at least 3 independent experiments. *P < .05, **P < .01, ***P < .001.
CCL2 promotes chemotaxis and migration of endothelial cells in vitro. (A) HUVECs were stained with an anti-CCR2 antibody (blue histogram) or isotype control (red histogram) and analyzed by FACS. (B) Migration of HUVECs toward conditioned medium of neoplastic MC (CM) was determined using a modified Boyden chamber assay. CM was pre-incubated with an antibody against CCL2 or an isotype control antibody (αISO) to determine the specificity of the observed effect. The number of migrated cells per field was counted and normalized to control level. (C-E) Migration of HUVECs in a scratch assay. The upper panel of (C) shows the scratch before and the lower panel after 24 hours of incubation with CM. CM was pre-incubated with an antibody against CCL2 or an isotype control antibody (αISO) as described above. The number of cells in the scratch was counted and normalized to control level. (D) Respective numbers of mean ± SD of at least 3 independent experiments. (E) In similar experiments, conditioned medium from neoplastic MCs after transduction with 2 different short hairpin RNAs (shRNAs) targeting CCL2 (#1 and #2) or an NTC was used. Results represent the mean ± SD of at least 3 independent experiments. *P < .05, **P < .01, ***P < .001.
Identification of CCL2 as critical MC-derived mediator in an in vivo NSG mouse model of mastocytosis
The impact of CCL2 on angiogenesis and tumor growth in vivo was assessed in a xenotransplantation model using NSG mice. Subcutaneous injection of HMC-1 cells with or without knockdown of CCL2 resulted in the formation of tumors within 23 days. Tumor volumes were measured between day 23 and 34 and showed a significant difference between CCL2-expressing and CCL2-knockdown cells (Figure 4A). After 34 days, tumors were isolated and analyzed for weight and microarchitecture. Tumors of HMC-1 cells after CCL2 knockdown were significantly lighter in comparison with the controls (Figure 4B). In addition, tumors showed a different microarchitecture after knockdown of CCL2 with a reduction in necrotic areas and leukocyte infiltration, a lower proliferation rate as determined by staining with Ki-67, and less tumor stroma (Figure 4C-G). In particular, CAB staining showed a striking difference in collagen deposition in the tumors (Figure 4E,H), and immunohistochemistry for von Willebrand factor indicated a reduced microvessel density after knockdown (Figure 4F,I). Overall, silencing of CCL2 expression in neoplastic MCs resulted in a more benign tumor stroma with concomitant reduction of tumor cell proliferation and tumor mass in vivo.
Knockdown of CCL2 reduces tumor growth, fibrosis, and microvessel density in vivo. (A-B) HMC-1 cells transduced with an shRNA targeting CCL2 (RNAi, red [n = 6]) or an NTC (blue [n = 5]) were injected subcutaneously into NSG mice. (A) Calculated tumor volume over time and (B) measured tumor weight at day 34 after injection. (C-F) Sections prepared from paraffin-embedded tumors were stained with (C) hematoxylin and eosin, (D) Ki-67 indicating proliferation, (E) Chromotrope-aniline blue (CAB) indicating fibrosis, or (F) an antibody against von Willebrand factor staining microvessels. Representative pictures of tumors after CCL2 knockdown (RNAi, right subpanels) or controls (NTC, left subpanels) are shown. (G-I) Grading (scale of 0 to 4) of (G) inflammation and (H) fibrosis as well as (I) microvessel density of the tumors after CCL2 knockdown compared with controls (NTC). *P < .05, ***P < .001.
Knockdown of CCL2 reduces tumor growth, fibrosis, and microvessel density in vivo. (A-B) HMC-1 cells transduced with an shRNA targeting CCL2 (RNAi, red [n = 6]) or an NTC (blue [n = 5]) were injected subcutaneously into NSG mice. (A) Calculated tumor volume over time and (B) measured tumor weight at day 34 after injection. (C-F) Sections prepared from paraffin-embedded tumors were stained with (C) hematoxylin and eosin, (D) Ki-67 indicating proliferation, (E) Chromotrope-aniline blue (CAB) indicating fibrosis, or (F) an antibody against von Willebrand factor staining microvessels. Representative pictures of tumors after CCL2 knockdown (RNAi, right subpanels) or controls (NTC, left subpanels) are shown. (G-I) Grading (scale of 0 to 4) of (G) inflammation and (H) fibrosis as well as (I) microvessel density of the tumors after CCL2 knockdown compared with controls (NTC). *P < .05, ***P < .001.
Primary MCs in systemic mastocytosis express CCL2
To study the significance of our findings in human disease, CCL2 serum levels were measured in a cohort of patients with mastocytosis (Table 1). Patients with mastocytosis showed significantly higher levels of CCL2 (450.9 ± 364.9 pg/mL [mean ± SD]; n = 48) compared with controls (282 ± 92.9 pg/mL [mean ± SD]; P = .0027) (Figure 5A). To investigate whether CCL2 was expressed by neoplastic MCs, we examined BM sections obtained from patients with SM. As assessed by immunohistochemistry, tryptase-positive spindle-shaped neoplastic MCs were found to react with anti-CCL2 antibody (Figure 5C-D). Finally, highly purified (fluorescence-activated cell sorting) primary neoplastic MCs of patients with advanced SM (n = 2) expressed substantial amounts of CCL2 by quantitative PCR (ASM, 1783 ± 26; MCL, 337 ± 84; CCL2 mRNA levels percent of ABL [mean ± SD]).
CCL2 is a prognostic biomarker in mastocytosis. (A-B) CCL2 serum levels of mastocytosis patients compared with (A) an age- and sex-matched control cohort and (B) between subgroups of mastocytosis (cutaneous mastocytosis [CM], mastocytosis in the skin [MIS], ISM, advanced SM). (C-D) Serial sections prepared from paraffin-embedded BM of a patient with SM were stained by immunohistochemistry using (C) an anti-tryptase antibody (pink staining) or (D) an anti-CCL2 antibody (brown staining). Neoplastic MC infiltrates were found to stain positive for CCL2. Lower subpanels show a higher magnification (original magnification ×400) of the upper subpanels (original magnification ×100). (E-F) Kaplan-Meier plot for overall survival of mastocytosis patients stratified according to the upper reference range of CCL2 serum level of 468 pg/mL. The difference in the probability of survival was significant for (E) all mastocytosis patients and (F) the subgroup of patients with ISM. *P < .05, **P < .01
CCL2 is a prognostic biomarker in mastocytosis. (A-B) CCL2 serum levels of mastocytosis patients compared with (A) an age- and sex-matched control cohort and (B) between subgroups of mastocytosis (cutaneous mastocytosis [CM], mastocytosis in the skin [MIS], ISM, advanced SM). (C-D) Serial sections prepared from paraffin-embedded BM of a patient with SM were stained by immunohistochemistry using (C) an anti-tryptase antibody (pink staining) or (D) an anti-CCL2 antibody (brown staining). Neoplastic MC infiltrates were found to stain positive for CCL2. Lower subpanels show a higher magnification (original magnification ×400) of the upper subpanels (original magnification ×100). (E-F) Kaplan-Meier plot for overall survival of mastocytosis patients stratified according to the upper reference range of CCL2 serum level of 468 pg/mL. The difference in the probability of survival was significant for (E) all mastocytosis patients and (F) the subgroup of patients with ISM. *P < .05, **P < .01
CCL2 is a marker of advanced mastocytosis
CCL2 serum levels in advanced SM were significantly higher compared with indolent SM and cutaneous mastocytosis (Figure 5B). CCL2 levels showed only modest correlation to serum tryptase levels, MC infiltration in the BM, KIT D816V allele burden, and BM fibrosis (supplemental Figure 2). In addition, no difference in CCL2 levels was observed when comparing patients with or without mediator-related symptoms (supplemental Figure 2). We next examined the impact of serum CCL2 levels on survival and used the upper reference range as a cutoff to separate two prognostically distinct groups. Mastocytosis patients with CCL2 levels above 468 pg/mL (n = 15) showed a median survival of 2.8 years, whereas the median survival was not reached in the group of patients with CCL2 levels below 468 pg/mL (n = 33; log-rank test, P < .001; Figure 5E). In the group of ISM patients, a significant survival difference was observed (P = .0011; Figure 5F). Together, our data show that high CCL2 is associated with advanced disease and poor survival in mastocytosis.
Discussion
The activating KIT D816V mutation has been implicated in the pathogenesis of SM and represents a minor diagnostic SM criterion. However, mechanisms underlying the biological effects of the mutated KIT receptor are still not fully understood. The important interplay of neoplastic (stem) cells and altered tumor microenvironment is well known in solid cancers and has also been addressed in classical MPNs. Interactions of neoplastic cells and their microenvironment contribute to therapy resistance, tumor stem cell homeostasis, metastasis, aggressive tumor growth, and paraneoplastic symptoms, among other things. Data focusing on the role of the microenvironment in SM are limited. Cytokines have been shown to affect the microenvironment in SM and are considered a major driver of disease progression and clinical symptoms in JAK2 V617F+ MPNs.31
KIT D816V activates various downstream signaling pathways that modulate gene expression and drive increased cytokine expression. We identified NF-κB signaling to be critical for KIT D816V–dependent expression of CCL2. CCL2 is a well-known NF-κB target gene.58,59 However, NF-κB has not been prominently discussed as a major signaling pathway downstream of KIT D816V in SM. Tanaka et al60 showed constitutive activation of NF-κB signaling in KIT D816V+ HMC-1 cells. These data are in line with our observation of the pivotal role of NF-κB signaling for CCL2 expression and suggest a pathophysiological relevance for NF-κB signaling in SM. In addition, targeting strategies for NF-κB in SM have already been suggested.60
Our in vitro data clearly show that KIT D816V triggers expression of CCL2. Although the mutation is found in >90% of SM patients, marked differences in CCL2 serum levels between patients were observed. The number of MCs, the expression of CCL2 in other (neoplastic) cells, or additional genetic mutations in MCs might influence the CCL2 serum level. First, we observed only a loose correlation between CCL2 and surrogate parameters of the MC burden, such as serum tryptase and the degree of MC infiltration in the BM. Second, several cell types are capable of expressing CCL2, and increased CCL2 has been described in various myeloid neoplasms.4,42 Multilineage involvement of KIT D816V being present not only in neoplastic MCs but also in clonal myeloid cells is a common finding in advanced SM.61 Thus, it is likely that clonal non-MCs contribute to increased CCL2 levels, especially in SM-AHN. Third, recent sequencing studies in patients with advanced SM have shown several additional somatic mutations in addition to KIT D816V.62 Additional studies that investigate the mechanisms of cooperation between KIT D816V and additional oncoproteins to elucidate their joint effect on cytokine expression, microenvironment alterations, and pathogenesis in SM are quite interesting.
With regard to the clinical relevance of CCL2 serum measurement, we observed increased CCL2 levels in patients with mastocytosis and the highest levels in patients with advanced SM. Thus, measurement of CCL2 might contribute to risk assessment in SM. Comparable data have been published for other cytokines.28 The observed difference in overall survival might be partly explained by the enrichment of patients with advanced SM in the group with high CCL2 levels. However, a significant survival difference was also observed in the group of ISM patients. Thus, our data indicate an association of high CCL2 levels with advanced disease and poor survival in mastocytosis. Nevertheless, the clinical significance of CCL2 measurement in addition to established risk factors as well as other cytokine profiles needs to be assessed in forthcoming studies that include larger numbers of patients.
We found that MC-derived CCL2 promotes migration of endothelial cells and alterations of the tumor microenvironment in SM in vitro and in vivo. In particular, tumors showed vascularization and fibrosis, and profound effects on the microenvironment were observed after knockdown of CCL2. Thus, valuable data regarding the interaction of neoplastic MC and tumor microenvironment could be obtained. Currently, other human MC lines, such as the recently described ROSA and MCPV, are being investigated in our laboratory.49,63 Preliminary data indicate that these MC lines produce CCL2-positive tumors as well. Additional in vivo studies on the interaction of neoplastic MCs and BM cells are warranted to further increase our understanding of the pathophysiologic role of CCL2 in SM.
The concept of targeting CCL2 in SM is in line with data from several studies showing a significant increase in survival and inhibition of metastasis by targeting CCL2 in solid cancers.35,38-40 The effect can be partly explained by direct effects on endothelial cells and fibroblasts. In addition, targeting of CCL2 also reduces infiltration with tumor-associated macrophages and thus indirectly affects other cells of the tumor microenvironment.64 Although targeting of CCL2 showed promising results in various tumor models, one recent study raised concerns about the acceleration of breast cancer metastasis by promoting angiogenesis after cessation of CCL2 inhibition.41 This further strengthens the important role of CCL2 in tumor progression but needs to be carefully addressed when applying this targeting strategy to the patients. Our data indicate that targeting of cytokines, including CCL2, might also be a promising strategy in advanced SM, complementary to the direct targeting of KIT D816V.
In summary, the chemokine CCL2 promotes alterations of the tumor microenvironment in SM in vitro and in vivo. High serum levels of CCL2 in patients correlate with advanced disease and poor survival. Our data imply that CCL2 is another important player in the complex interplay of neoplastic MCs and BM microenvironment and thus a potential therapeutic target in SM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Eva Schrefl and Sophie Frank (Department of Laboratory Medicine), Günther Hofbauer and Andreas Spittler (Cell Sorting Core Facility), and Sabine Rauscher and Marion Gröger (Imaging Core Facility) for technical support. They also thank Helmuth Haslacher, Thomas Perkmann, and Peter Quehenberger (Department of Laboratory Medicine) for contributing serum samples for the control cohort and Gertrude Krainz for proofreading the manuscript (Department of Pathology, Medical University of Vienna, Vienna, Austria for all).
This study was supported by Austrian National Science Fund (FWF) projects P26079-B13 and F4704-B20. L.K. has received funding from FWF projects P26011 and P29251 and MSCA-ITN-2015-ETN ALKATRAS, No. 675712.
Authorship
Contribution: G.G., N.W., A.B., K.S., G.E., S.R., J.Z., L.K., V.S., M.M., and G.H. performed in vitro and in vivo experiments and analyzed the data; G.G., A.-I.S, T.P.-K., L.M., W.R.S., P.V., and G.H. obtained and analyzed clinical data; G.G., P.V., M.M., and G.H. designed the study and wrote the paper; and all authors revised and approved the manuscript.
Conflict-of-interest disclosure: P.V. served as a consultant in a global Novartis trial investigating the effects of midostaurin in patients with advanced systemic mastocytosis and received honoraria and research grants from Novartis, Blueprint, and Deciphera. G.H. and W.R.S. received honoraria from Novartis. The remaining authors declare no competing financial interests.
Correspondence: Gregor Hoermann, Department of Laboratory Medicine, Medical University of Vienna, Waehringer Guertel 18-20, Leitstelle 5H, A-1090 Vienna, Austria; e-mail: gregor.hoermann@meduniwien.ac.at.

![Figure 4. Knockdown of CCL2 reduces tumor growth, fibrosis, and microvessel density in vivo. (A-B) HMC-1 cells transduced with an shRNA targeting CCL2 (RNAi, red [n = 6]) or an NTC (blue [n = 5]) were injected subcutaneously into NSG mice. (A) Calculated tumor volume over time and (B) measured tumor weight at day 34 after injection. (C-F) Sections prepared from paraffin-embedded tumors were stained with (C) hematoxylin and eosin, (D) Ki-67 indicating proliferation, (E) Chromotrope-aniline blue (CAB) indicating fibrosis, or (F) an antibody against von Willebrand factor staining microvessels. Representative pictures of tumors after CCL2 knockdown (RNAi, right subpanels) or controls (NTC, left subpanels) are shown. (G-I) Grading (scale of 0 to 4) of (G) inflammation and (H) fibrosis as well as (I) microvessel density of the tumors after CCL2 knockdown compared with controls (NTC). *P < .05, ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/3/10.1182_blood-2016-09-739003/4/m_blood739003f4.jpeg?Expires=1766532685&Signature=T9KTOACYv1P6rHFtzXjikfqPNKljdl0G5jBeGcfWSHPKZrgKwcQ~ZfGgoAIrS8CSrXZYugXY7gUoLImRX2J~R-69Qu62l3mwechwKfvYG8aJAnmjRWnEUF0iP8DR6gaR7Tv0WB4C1plHREcxR7rjvrP2qVP02pTdwhzdWd-W6CbZPy8dNb5625JNcW1CRiJsB373yM9psx~DqrSR8YyvTOX3MOtLrqe7hW2N3VyLIrQzCbrfWGJ3a18W1gZVjr-Rn2v7S5lw~8gFhoQPefvjOJInml8iSpLeLl-LozonkBB2We4-cUJjxGrP3gE8L8on~9iM~SKXOV6LLOXeRxOX6Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. CCL2 is a prognostic biomarker in mastocytosis. (A-B) CCL2 serum levels of mastocytosis patients compared with (A) an age- and sex-matched control cohort and (B) between subgroups of mastocytosis (cutaneous mastocytosis [CM], mastocytosis in the skin [MIS], ISM, advanced SM). (C-D) Serial sections prepared from paraffin-embedded BM of a patient with SM were stained by immunohistochemistry using (C) an anti-tryptase antibody (pink staining) or (D) an anti-CCL2 antibody (brown staining). Neoplastic MC infiltrates were found to stain positive for CCL2. Lower subpanels show a higher magnification (original magnification ×400) of the upper subpanels (original magnification ×100). (E-F) Kaplan-Meier plot for overall survival of mastocytosis patients stratified according to the upper reference range of CCL2 serum level of 468 pg/mL. The difference in the probability of survival was significant for (E) all mastocytosis patients and (F) the subgroup of patients with ISM. *P < .05, **P < .01](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/3/10.1182_blood-2016-09-739003/4/m_blood739003f5.jpeg?Expires=1766532685&Signature=3I9tjIbxlFwNOwSNtRKgT0rUAXSwkvjRAVubz5hwUJ6dVJLAQoo6vjCHft~HiJ~jRMYpn4ZFoeapRPVUydVrqCFyxc5BLSK0BApgyYIppzky7Fqj5FkireOJMoBxn8gYzyNblG6FRGDGKaY9lA4JWFfBaSHsst406FE0SuYlV-HSc5KF8WNaqDAG7~ZDSYwJERXJYRXptdG0oxniVE2b4ZldjxJ4RTHijpLS71bz95xRjlBEJXy98RPSI~Wsxr1kFYfJA9UCNrq7focU7S0HdAVQiF-ul-EWyM8Naqs3dRUQ8hdo58PRPQ-18XhClA3uvL-Z6aPNqQYO86h3xqCUAQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 4. Knockdown of CCL2 reduces tumor growth, fibrosis, and microvessel density in vivo. (A-B) HMC-1 cells transduced with an shRNA targeting CCL2 (RNAi, red [n = 6]) or an NTC (blue [n = 5]) were injected subcutaneously into NSG mice. (A) Calculated tumor volume over time and (B) measured tumor weight at day 34 after injection. (C-F) Sections prepared from paraffin-embedded tumors were stained with (C) hematoxylin and eosin, (D) Ki-67 indicating proliferation, (E) Chromotrope-aniline blue (CAB) indicating fibrosis, or (F) an antibody against von Willebrand factor staining microvessels. Representative pictures of tumors after CCL2 knockdown (RNAi, right subpanels) or controls (NTC, left subpanels) are shown. (G-I) Grading (scale of 0 to 4) of (G) inflammation and (H) fibrosis as well as (I) microvessel density of the tumors after CCL2 knockdown compared with controls (NTC). *P < .05, ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/3/10.1182_blood-2016-09-739003/4/m_blood739003f4.jpeg?Expires=1766871324&Signature=MdgjVCZ5FX65d7jJ~rbUBPgCgg8Sv2PRvHIcVuW3P-gAAK2MkjWZGANtzfG7YAL5tyPYa-SvGrNbbtuxYEFj1M72KsGQ7lmZWg5oc48-dteMJzMGYEXxTxgYiBrjHYwa0oftiB53~mz2uGjIouUDRiDxlcxhQybRFIEBIo7IhHQGGJBRQ8sBqyAVTxnmyEXbK0LJIFVJdti1cX0NfAPlGhjmf5yZj92kWOsvR~wEFNcvg61OFmj9-Ysz39vS6psLc-Vb~Eylao7xxpTRA~BC2G~yiWDJ3lE4rl3GrDkC88pQB~nsoDBDhhltSIZp0F1W347Nw8MlJeVF1eNsGLlrSQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. CCL2 is a prognostic biomarker in mastocytosis. (A-B) CCL2 serum levels of mastocytosis patients compared with (A) an age- and sex-matched control cohort and (B) between subgroups of mastocytosis (cutaneous mastocytosis [CM], mastocytosis in the skin [MIS], ISM, advanced SM). (C-D) Serial sections prepared from paraffin-embedded BM of a patient with SM were stained by immunohistochemistry using (C) an anti-tryptase antibody (pink staining) or (D) an anti-CCL2 antibody (brown staining). Neoplastic MC infiltrates were found to stain positive for CCL2. Lower subpanels show a higher magnification (original magnification ×400) of the upper subpanels (original magnification ×100). (E-F) Kaplan-Meier plot for overall survival of mastocytosis patients stratified according to the upper reference range of CCL2 serum level of 468 pg/mL. The difference in the probability of survival was significant for (E) all mastocytosis patients and (F) the subgroup of patients with ISM. *P < .05, **P < .01](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/3/10.1182_blood-2016-09-739003/4/m_blood739003f5.jpeg?Expires=1766871324&Signature=bmCRdh~vRRvzM-68piud8YS1y-Eo4tTls2h~j3AUQ4iPO1ro1uAa1kFtTPNpPWT0ZIbb3TevNUnxTbgo26vS8x4utyugGY0drguVsCn~axmbVCdR6KchNbYSKL7h2xBlWxDa7VjOsObShlhNTo4Py6S51~BVNZLTKI80TJun~1tM-I6K4VueMCfGX12k3fKsKx7HOPTVvVzs1~DVr1NRvxG1gNSnZsi61Fqk~1raE6oImCJYUMmzcVYmMWBl-cQR06yHWVVq6VxWbthlSD-O0hnWflS4G6I9ZTdfEKNJVRRVZQeVn4TLVQYkrAPRo0qmRz1PZR9xiQgFpdNyFXrbvg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)