Key Points
TR4 and TR2 execute distinct functions during embryogenesis and erythroid differentiation.
TR4 promotes erythroid proliferation and maturation.
Abstract
The orphan nuclear receptors TR4 (NR2C2) and TR2 (NR2C1) are the DNA-binding subunits of the macromolecular complex, direct repeat erythroid-definitive, which has been shown to repress ε- and γ-globin transcription during adult definitive erythropoiesis. Previous studies implied that TR2 and TR4 act largely in a redundant manner during erythroid differentiation; however, during the course of routine genetic studies, we observed multiple variably penetrant phenotypes in the Tr4 mutants, suggesting that indirect effects of the mutation might be masked by multiple modifying genes. To test this hypothesis, Tr4+/− mutant mice were bred into a congenic C57BL/6 background and their phenotypes were reexamined. Surprisingly, we found that homozygous Tr4 null mutant mice expired early during embryogenesis, around embryonic day 7.0, and well before erythropoiesis commences. We further found that Tr4+/− erythroid cells failed to fully differentiate and exhibited diminished proliferative capacity. Analysis of Tr4+/− mutant erythroid cells revealed that reduced TR4 abundance resulted in decreased expression of genes required for heme biosynthesis and erythroid differentiation (Alad and Alas2), but led to significantly increased expression of the proliferation inhibitory factor, cyclin dependent kinase inhibitor (Cdkn1c). These studies support a vital role for TR4 in promoting erythroid maturation and proliferation, and demonstrate that TR4 and TR2 execute distinct, individual functions during embryogenesis and erythroid differentiation.
Introduction
TR4 (NR2C2), a reportedly ubiquitous orphan nuclear receptor, along with its heterodimeric binding partner TR2 (NR2C1), were identified more than a decade ago as the DNA-binding components of the macromolecular protein complex, direct repeat erythroid-definitive.1 TR2 and TR4 can bind, as both homo- and heterodimers, with high affinity to the direct repeat nuclear receptor consensus sites of both the ε- and γ-globin promoters in human erythroid cells and to the homologous embryonic/fetal εy- and βh1 gene promoters in mouse erythroid cells.1-7 Because elevated expression of γ-globin has been found to be clinically beneficial to patients with β-thalassemia8 and sickle cell disease,9 γ-globin induction is able to compensate for the lack of β-globin gene synthesis in the case of β-thalassemia, and in sickle cell disease γ-globin synthesis and formation of hemoglobin (Hb) F (α2γ2 Hb tetramers) interrupts HbS sickle polymer formation generated by the mutant adult β-globin gene.1,9-13 Therefore, concerted efforts are under way to analyze pharmacological or genetic interventional strategies to inactivate the multiple γ-globin repressors that have been identified to date in an effort to stimulate more abundant γ-globin expression in the definitive, adult erythroid cells of sickle cell and β-thalassemia patients.6,11,14
Previous studies from our laboratory have shown that compound conditional deletion of the Tr2/Tr4 genes leads to increased εy- and βh1-globin and induction of transgenic human ε- and γ-globin expression in mice.3,5 However, these mice also had fewer differentiated erythroid cells and were not born in the expected Mendelian ratios, suggesting that TR2 and TR4 have physiological functions during erythroid development as well as possibly other undisclosed embryonic phenotypes not previously reported.
The phenotypic characteristics of global Tr4−/− mutant knockout mice were described previously, and these animals exhibited reductions in female reproduction, reduced body size, and abnormal behavioral patterns, but hematologic defects were not detected.3,15-17 Furthermore, no phenotypes were noted in Tr2−/− mutants.3,18 Compound Tr2−/−:Tr4−/− embryos exhibited preimplantation lethality, precluding analysis of their function in erythroid cells, originally suggesting that TR2 and TR4 act in a genetically redundant manner to regulate biological functions that are critical for embryonic development and survival.3 However, mixed compound Tr2+/−:Tr4−/− mice exhibited greater γ-globin derepression (in animals bearing a wild-type [WT] human β-globin YAC) than did Tr2−/−:Tr4+/− animals, indicating that TR4 might have a more potent biological influence than TR2 in erythroid cells.3
To examine the physiological effects of TR4 loss of function (LOF) in the absence of any potential complications from analysis in a mixed genetic background, and to elucidate molecular functions during erythroid differentiation, we backcrossed the Tr4+/− mice for more than 7 generations to generate congenic C57BL/6 animals. Surprisingly, and in contrast to a previous report,15 Tr4−/− mice do not survive gestation, but die before embryonic day 9.5 (E9.5), thereby demonstrating that this orphan nuclear receptor plays an uncompensated role in early embryonic development. Because Tr4−/− mice were embryonic lethal before the onset of hematopoiesis, we investigated whether erythroid phenotypes might be manifested in Tr4+/− animals. These studies revealed that, even in Tr4+/− mutants, erythroid differentiation and proliferation were profoundly affected. TR4 candidate target genes that regulate erythroid differentiation and proliferation were identified by analyzing published human RNA sequencing (RNA-seq) data,19 and the homologous expression alterations in murine mutants were validated by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) analysis of flow-sorted erythroid cells. These experiments demonstrate that not only does TR4 act in a nonredundant fashion with TR2 during embryonic development, but also plays a critical and previously undiscovered role in erythroid differentiation and proliferation.
Materials and methods
Mice
The generation of Tr4 mutant mice through targeted deletion of the DNA-binding domain in embryonic stem cells followed by breeding into a mixed 129/SvJ genetic background was previously reported.3 Tr4+/− mice were then backcrossed to C57BL/6 (CD45.2) mice for more than 7 generations. Genotyping was performed by PCR of tail biopsies or yolk sacs (oligos in Table 1). All animal experiments were reviewed and approved by the University Committee on Use and Care of Animals at the University of Michigan.
Flow cytometry
For viability studies, timed matings were generated by intercrossing Tr4+/− mice, where the presence of a copulation plug was considered E0.5. At E13.5 or E15.5, fetal livers were isolated in phosphate buffered saline supplemented with 2% fetal bovine serum, aspirated by syringe through a 21-gauge needle, and filtered through a 0.22-μm filter to generate a single cell suspension. Adult spleens were isolated, weighed, and a single-cell suspension was generated. Bone marrow was isolated from the femurs of adult (6 to 8 weeks old) mice in the same manner as previously reported.20
Flow cytometry was performed on an LSRFortessa analyzer (BD Biosciences).21 To analyze erythroid differentiation, cells were stained with α-CD71(R17217), α-TER119 (TER119), and α-CD44 (IM7) as previously described.22 To analyze myeloid and B-cell lineages, first, the red blood cells (RBCs) were lysed by incubation in 0.24 M NH4Cl for 5 minutes at room temperature and then stained with α-CD11b (M1/70) and α-Gr1 (RB6-8C5) for myeloid cells; B-cell lineages were stained with α-B220 (RA3-6B2) and α-CD19 (6D5). Cell death was assessed by Annexin V,23 and proliferation was quantified by α-Ki67 (BD Biosciences) and 4′,6-diamidino-2-phenylindole (DAPI) according to the manufacturer’s protocol. Peripheral blood was stained with o-thiazole orange using standard protocols to assess the percentage of reticulocytes.13 For all flow cytometry experiments, antibodies were from Biolegend unless otherwise stated; data were analyzed using FlowJo 10.2.
Colony-forming assays
Colony assays were performed with lineage-depleted bone marrow cells according to the manufacturer’s protocol using MethoCult (Stem Cell Technologies, M3334). Colonies were analyzed on a stereoscopic microscope (SMZ1500; Nikon) and imaged with a Digital Sight DS-Ri1 camera (Nikon) and NIS Elements software (Nikon).
qRT-PCR
CD71+TER119+-labeled bone marrow cells were fluorescence-activated cell sorter (FACS)-purified using a FACSAriaII (BD Biosciences),21 and RNA was isolated by Trizol (Invitrogen) extraction using the manufacturer’s protocols. Complementary DNA was generated using SuperScript IV First-Strand Synthesis (Invitrogen). qRT-PCR was performed using an ABI StepOnePlus (Applied Biosystems) with FastSybr Green Mastermix (Applied Biosystems). Levels of the Oaz1 transcript were used as the normalization control for all qRT-PCR experiments.24 The oligos used for qRT-PCR analysis (Alas2,25 Alad,26 βh1-globin,3 βmajor-globin,3 Cdkn1c,27 εY-globin,3 Tr2,3 and Tr43 ) are listed in Table 1.
Complete blood counts
Peripheral blood was collected from the submandibular vein using EDTA-treated tubes and analyzed with a Hemavet (Drew Scientific).
Histological analysis
Whole bone marrow and stage-specific FACS-purified erythroid cells were cytospun at a concentration of 5 × 105 to 1 × 106 cells/sample followed by Wright-Giemsa (Sigma, WG16) staining performed according to the manufacturer’s protocol. Peripheral blood smears were stained with neutral benzidine followed by Wright-Giemsa staining.28 Images were acquired using an Olympus BX-51 upright light microscope with 60× and 100× oil immersion objective lenses, a DP-70 high-resolution digital camera, and DP-70 Controller software.
Statistical analysis
Statistical significance was determined by Student t test, where P ≤ .05 was considered significant.
Results
Tr4 null mice are embryonic lethal
Previous studies from our laboratory and others have examined Tr4−/− mutant mice in a mixed genetic background with varying contributions from 129/SvJ, C57BL/6J (BL6), and CD1 mice.3,5,15-18 We reported that progeny are viable with variable growth and fertility defects, but the mutants appeared to lack any hematological abnormalities.3,5,15-17 Because genetic modifiers can powerfully affect phenotypes in a mixed genetic background, we generated Tr4 congenic mutant animals (C57BL/6). Following at least 7 generations of backcrossing, Tr4+/− × Tr4+/− intercrosses surprisingly yielded no Tr4−/− pups when progeny were weaned at postnatal day (P) 21 (Table 2), initially suggesting that Tr4−/− results in perinatal or embryonic lethality. To more precisely determine the time of Tr4−/− lethality, timed matings were performed and embryos were examined for viability (heartbeat) at various developmental stages. Surprisingly, viable homozygous mutant embryos were recovered only beginning at E7.0, but not at later embryonic stages, indicating that Tr4−/− lethality occurs between implantation and E9.5 (Table 2). Erythropoiesis in the yolk sac begins at E7.5, but embryos do not exhibit lethality from oxygen deprivation until around E11.0 (for example, in Gata-1 or Klf1 LOF mutants).29-31 These data demonstrate that the Tr4−/− lethal deficiency during embryonic development is unlikely to be due to an erythroid defect.
Tr4+/− mice have defects in erythroid differentiation
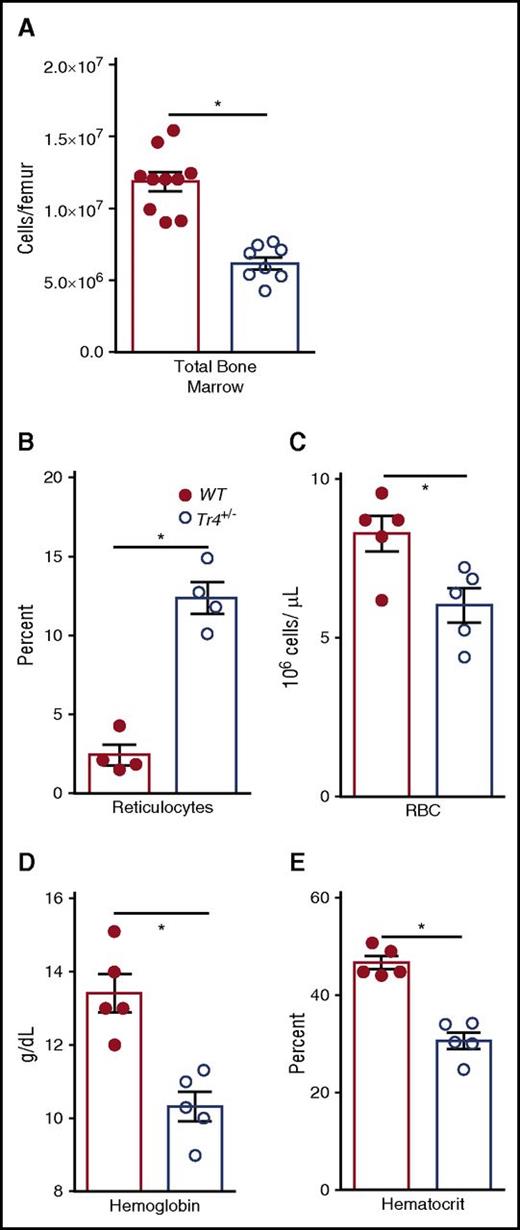
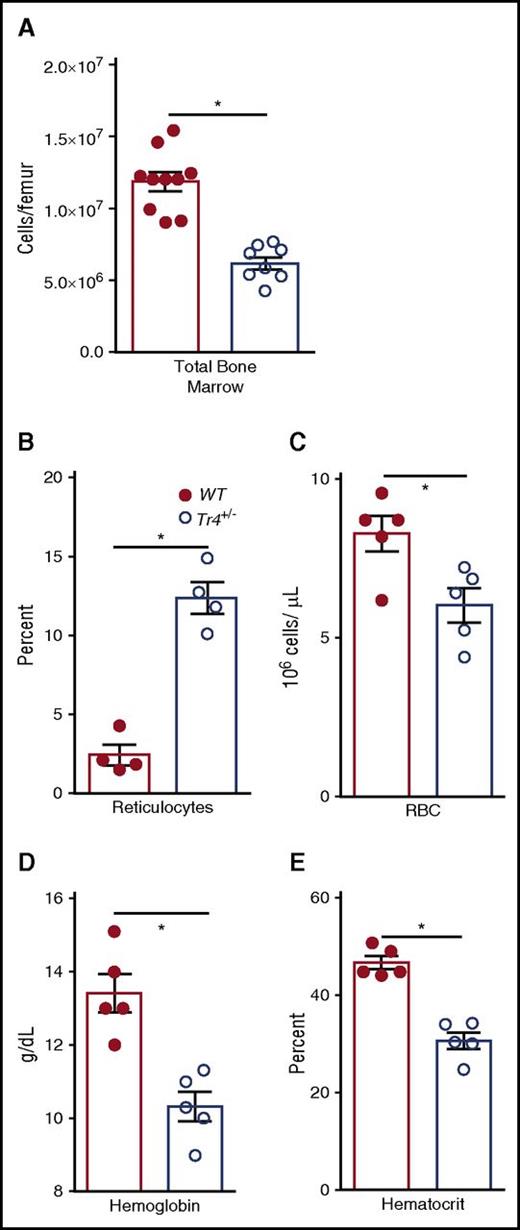
Because we were curious about the possible functions of TR4 in normal erythroid cell physiology, we examined hematopoietic differentiation in bone marrow of congenic Tr4+/− mutants and performed complete blood counts (CBCs) on the peripheral blood of 6- to 8-week-old Tr4 mutant and control WT mice (Figure 1; Table 3). Surprisingly, we observed a significant reduction in the total number of bone marrow cells in Tr4+/− mice (Figure 1A). CBCs revealed that Tr4+/− adult mice are anemic, have a significantly increased percentage of reticulocytes (Figure 1B), reduced RBC (Figure 1C) with lower overall Hb concentrations (Figure 1D) and reduced hematocrits (HCT, Figure 1E) when compared with their WT littermate controls. CBCs were also performed at 6 months of age; the decrease in RBC, Hb, and HCT appears to be present throughout adulthood (Table 3).
Tr4 heterozygous mutant mice are anemic. (A) The number of total bone marrow cells was quantified in WT (closed circles) and Tr4+/− (open circles) femurs. Peripheral blood was collected from 6- to 8-week-old WT (Tr4+/+; closed circles) or heterozygous mutant (Tr4+/−; open circles) B6 congenic mice and then stained with o-thiazole orange to determine the percentage of (B) reticulocytes and CBCs to analyze the (C) concentration (RBC), (D) Hb, and (E) hematocrit in triplicate samples. *P ≤ .05 as determined by Student t test comparing Tr4+/− with WT.
Tr4 heterozygous mutant mice are anemic. (A) The number of total bone marrow cells was quantified in WT (closed circles) and Tr4+/− (open circles) femurs. Peripheral blood was collected from 6- to 8-week-old WT (Tr4+/+; closed circles) or heterozygous mutant (Tr4+/−; open circles) B6 congenic mice and then stained with o-thiazole orange to determine the percentage of (B) reticulocytes and CBCs to analyze the (C) concentration (RBC), (D) Hb, and (E) hematocrit in triplicate samples. *P ≤ .05 as determined by Student t test comparing Tr4+/− with WT.
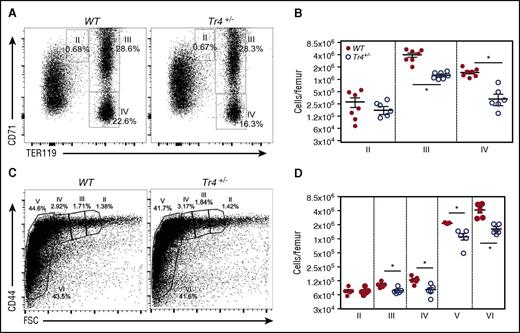
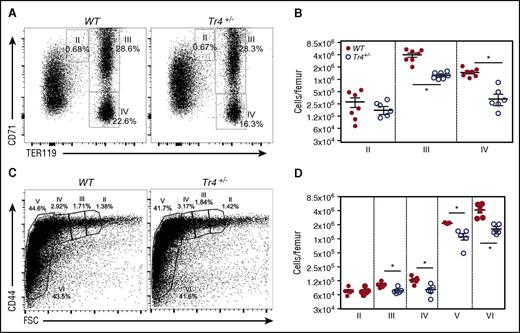
Next, we more closely examined the stage(s) at which erythroid differentiation blocks arise in bone marrow. Whole bone marrow single-cell suspensions were analyzed by α-CD71/α-TER119 staining, which separates erythroid cells into 4 distinct quadrants based on developmental age, from I (earliest) to IV (most mature).32-34 Additionally, cells were analyzed according to their α-CD44/forward scatter (FSC) profiles (Figure 2C-D), where differentiation occurs from stages II through VI.22 Analyses of adult bone marrow cells revealed a defect in erythroid differentiation (stage III, CD71+TER119+, and stage IV, CD71−TER119+) in Tr4+/− mutants (Figure 2A-B). Additionally, α-CD44/FSC profiling was performed as previously reported (Figure 2C)22 ; these analyses confirmed a significant reduction in erythroid differentiation (Figure 2D). These data provide clear evidence that TR4 promotes and is required for adult erythroid cell differentiation.
Adult Tr4 heterozygous mutant mice exhibit impaired erythropoiesis. (A) Flow cytometric analysis of bone marrow cells recovered from 6- to 8-week-old WT and Tr4+/− femurs stained with α-CD71 and α-TER119 antibodies. The percentage of cells within each gate is indicated on the flow plots. (B) The absolute number of cells within each gate was quantified in WT (closed circles) and Tr4+/− (open circles) mice at each stage (II, III, and IV) of CD71/TER119-monitored erythroid differentiation.32,33 (C) Flow cytometric analysis of total bone marrow cells from 6- to 8-week-old WT and Tr4+/− femurs stained with α-CD44 vs FSC as another indication of erythroid differentiation (II-VI).58 The percent of cells within each gate is shown on a representative flow plot. (D) The absolute number of cells in each gate. *P ≤ .05 as determined by Student t test comparing Tr4 heterozygous mutant with WT bone marrow cells.
Adult Tr4 heterozygous mutant mice exhibit impaired erythropoiesis. (A) Flow cytometric analysis of bone marrow cells recovered from 6- to 8-week-old WT and Tr4+/− femurs stained with α-CD71 and α-TER119 antibodies. The percentage of cells within each gate is indicated on the flow plots. (B) The absolute number of cells within each gate was quantified in WT (closed circles) and Tr4+/− (open circles) mice at each stage (II, III, and IV) of CD71/TER119-monitored erythroid differentiation.32,33 (C) Flow cytometric analysis of total bone marrow cells from 6- to 8-week-old WT and Tr4+/− femurs stained with α-CD44 vs FSC as another indication of erythroid differentiation (II-VI).58 The percent of cells within each gate is shown on a representative flow plot. (D) The absolute number of cells in each gate. *P ≤ .05 as determined by Student t test comparing Tr4 heterozygous mutant with WT bone marrow cells.
Tr4+/− mice do not exhibit splenomegaly
Because Tr4+/− mice are moderately anemic, we examined the mutant animals for extramedullary erythropoiesis. Spleen size and weight appeared normal, and the total number of cells per spleen was also comparable to WT controls (supplemental Figure 1A-C, available on the Blood Web site), indicating that Tr4 LOF does not result in splenomegaly. Erythropoiesis was analyzed using α-CD71/α-TER119 staining, and while a small but statistically significant increase was observed in the number of Tr4+/− stage III (supplemental Figure 1D-E) cells, this trend did not result in increased number of stage IV cells. Furthermore, we observed comparable numbers of colonies in colony forming assays in both the Tr4+/− and WT spleens (supplemental Figure 1F-G). These data suggest that extramedullary erythropoiesis is not increased in Tr4+/− mutant mice.
Tr4+/− mutant embryos have impaired fetal liver erythroid differentiation
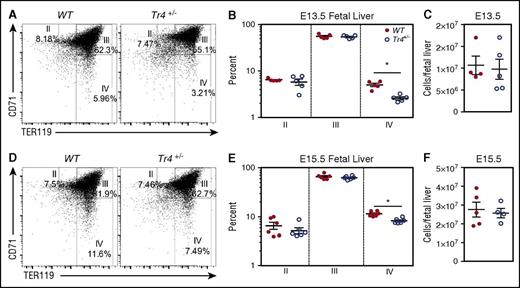
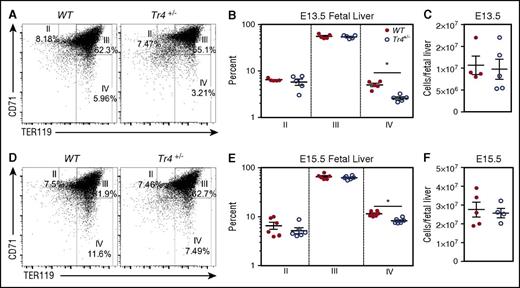
In order to examine whether the alterations detected in adult bone marrow are exhibited in fetal liver-derived erythroid cells we examined erythroid differentiation in the fetal livers of embryos. Fetal livers were isolated from E13.5 and E15.5 Tr4+/− mutant and WT mice, stained with α-CD71/α-TER119, and then examined for erythroid differentiation by flow cytometry (Figure 3). The percentage of stage IV (CD71−TER119+) erythroid cells was significantly diminished in Tr4+/− mice (compared with WT littermate controls) at both E13.5 and E15.5 (Figure 3A,B,D,E). However, no significant differences were observed in the earlier stages (II-III) of erythroid differentiation. The decrease observed did not appear to be due to changes in the total number of fetal liver cells (Figure 3C,F). These data indicate that TR4 plays an independent role that is uncomplemented by any other protein (eg, TR2) in erythroid cell differentiation, and is required for proper terminal erythroid maturation in the fetal liver.
Impaired erythroid differentiation in Tr4 heterozygous mutant fetal liver cells. Representative flow cytometry diagrams of fetal liver cells recovered from WT and Tr4+/− embryos at (A) E13.5 and (D) E15.5, stained with α-CD71 and α-TER119 antibodies. The percentage of cells within each gate (II-IV) is indicated on the plots. (B,E) Graph reporting the percentage of cells within each gate describing erythroid differentiation (II-IV) in (B) E13.5 (E) and E15.5 in WT (closed circles) and Tr4+/− (open circles) fetal liver cells.32,33 (C,F) Total cells per fetal liver were calculated for WT and Tr4+/− mice. *P ≤ .05 as determined by Student t test comparing Tr4+/− with WT.
Impaired erythroid differentiation in Tr4 heterozygous mutant fetal liver cells. Representative flow cytometry diagrams of fetal liver cells recovered from WT and Tr4+/− embryos at (A) E13.5 and (D) E15.5, stained with α-CD71 and α-TER119 antibodies. The percentage of cells within each gate (II-IV) is indicated on the plots. (B,E) Graph reporting the percentage of cells within each gate describing erythroid differentiation (II-IV) in (B) E13.5 (E) and E15.5 in WT (closed circles) and Tr4+/− (open circles) fetal liver cells.32,33 (C,F) Total cells per fetal liver were calculated for WT and Tr4+/− mice. *P ≤ .05 as determined by Student t test comparing Tr4+/− with WT.
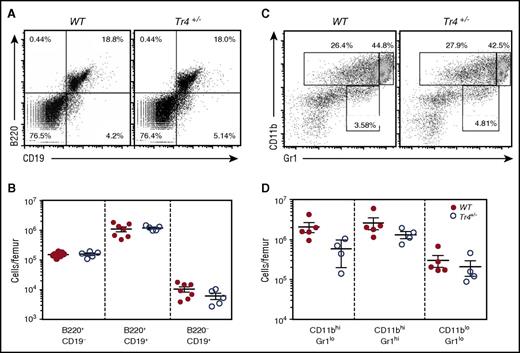
Tr4+/− mice have normal B- and myeloid cell differentiation
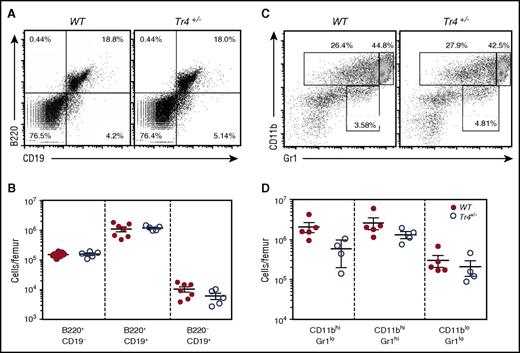
Because TR4 is broadly, if not ubiquitously, expressed,35 we also wished to determine whether or not other hematopoietic cell populations might be affected by the Tr4 LOF. Analysis of the B-cell lineage (following α-B220/α-CD19 staining, Figure 4A-B) and myeloid cells (α-CD11b/α-Gr1 staining, Figure 4C-D) revealed no statistically significant change in any of these populations, suggesting that the decreased bone marrow cellularity documented in Tr4+/− mice (Figure 1A) arises primarily as a consequence of the diminished number of erythroid and erythroid progenitor cells.
No significant differences in B- or myeloid cell populations in the adult bone marrow of Tr4+/−mice. (A,C) Representative flow cytometric diagram of total bone marrow cells recovered from 6- to 8-week-old WT (closed circles) or Tr4 heterozygous mutant (open circles) mice. RBCs were lysed and then the remaining bone marrow cells were stained with (A) α-B220 and α-CD19 for B-cell analysis or with (C) α-CD11b and α-Gr1 for myeloid cell analysis. The total number of cells per femur was quantified for the gates indicated in panels A and C. (B,D). No significant differences were observed by Student t test comparing Tr4 heterozygous mutant with WT bone marrow cells.
No significant differences in B- or myeloid cell populations in the adult bone marrow of Tr4+/−mice. (A,C) Representative flow cytometric diagram of total bone marrow cells recovered from 6- to 8-week-old WT (closed circles) or Tr4 heterozygous mutant (open circles) mice. RBCs were lysed and then the remaining bone marrow cells were stained with (A) α-B220 and α-CD19 for B-cell analysis or with (C) α-CD11b and α-Gr1 for myeloid cell analysis. The total number of cells per femur was quantified for the gates indicated in panels A and C. (B,D). No significant differences were observed by Student t test comparing Tr4 heterozygous mutant with WT bone marrow cells.
Erythroid morphology is unaltered in Tr4+/− mutants
Adult erythroid cell morphology was evaluated by staining of peripheral blood smears, whole bone marrow, and FACS-purified stage-specific erythroid cells by Wright-Giemsa staining (supplemental Figure 2). The morphology of peripheral blood smears of Tr4+/− mice appear normal compared with WT mice (supplemental Figure 2A-B′); similarly, whole bone marrow morphology appeared normal (supplemental Figure 2C-D′). Stage III (CD71+TER119+) and stage IV (CD71−TER119+) cells were FACS-purified, and stage III (supplemental Figure 2E-F) largely contained nucleated erythroid cells, whereas stage IV was composed of enucleated cells (supplemental Figure 2E-F). These studies suggest that TR4 likely does not affect erythroid cellular structure.
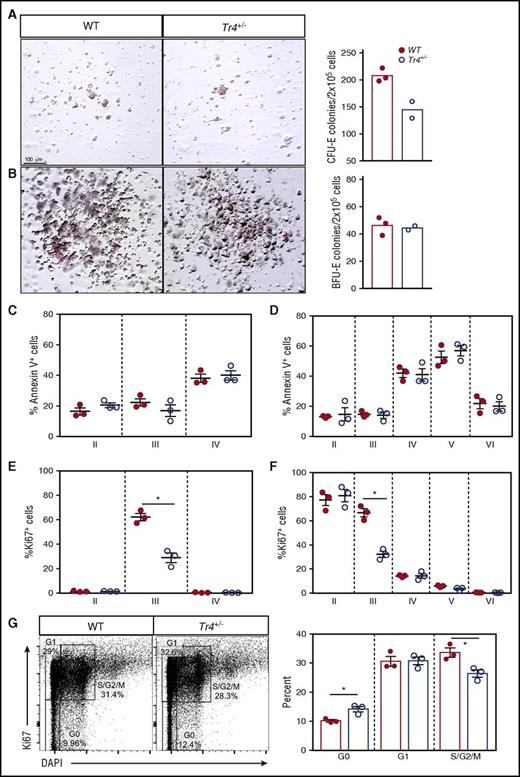
Reduced erythroid cell proliferation results in terminal erythropoiesis defects in Tr4+/− mutants
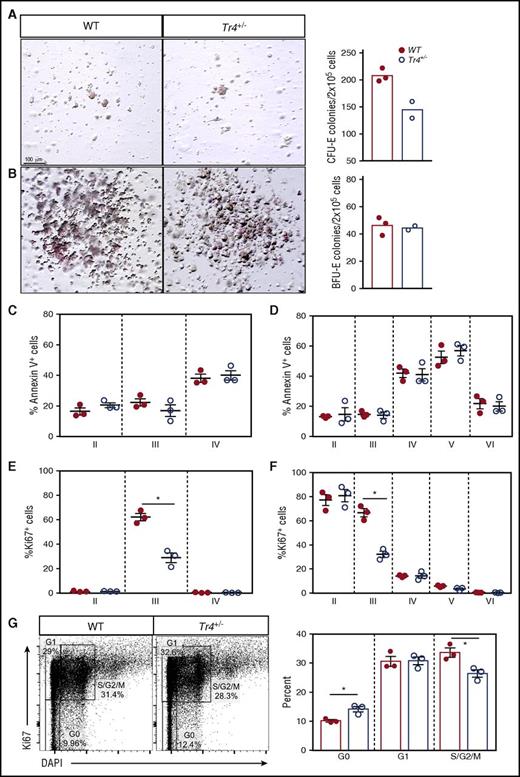
To identify the cellular processes resulting in impaired terminal erythroid differentiation observed in Tr4+/− mice, colony-forming assays, apoptosis, and proliferation were independently evaluated (Figure 5A-H). Using lineage-depleted bone marrow cells grown under erythroid-specific colony-forming conditions, Tr4+/− colonies yielded significantly fewer erythroid colony-forming units than WT, however; no difference in the number of burst-forming unit colonies was observed (Figure 5A-B). Although no statistically differences in apoptosis were observed at any stage of erythroid differentiation (Figure 5C-D), a significant reduction in the number of proliferating cells was observed (Figure 5E-F). To examine the proliferative defect in more detail, the percentage of cells in each stage of cell cycle (G0, G1, S/G2/M) was evaluated at stage III (CD71+TER119+; Figure 5G), the stage where we observe a decrease in proliferation. In Tr4+/− stage III cells, more were in a quiescent stage (G0) and fewer were in active mitosis (S/G2/M; Figure 5G). These data demonstrate that loss of one Tr4 allele has little effect on apoptotic cell death, but significantly reduces proliferation during erythroid cellular differentiation.
Tr4 heterozygous mutation results in decreased erythroid cell proliferation without altering apoptosis. Colony-forming assays were performed by plating lineage negative bone marrow cells in erythroid MethoCult (M3334; see “Materials and methods”), imaged, (A) the number of erythroid colony-forming unit (CFU-E) colonies quantified after 3 days and (B) burst-forming unit (BFU-E) colonies after 14 days. (C,D) Bone marrow cells were isolated from WT and Tr4+/− femurs and stained with α-CD71, α-TER119, α-CD44 (as in Figure 2), and Annexin V antibodies (to assess apoptosis), and the total percentage of cells within each gate were quantified. (E,F) Bone marrow cells were stained with α-CD71, α-TER119, α-CD44, and α-Ki67 antibodies (to assess proliferation), and the total percentage of cells within each gate was quantified. (G) Stage III (CD71+TER119+) erythroid cells were stained with α-Ki67 and DAPI antibodies. Gating for stages of proliferation are indicated on the left and the percentage of cells within each gate is graphed on the right. *P ≤ .05 as determined by Student t test comparing Tr4 heterozygous mutant with WT bone marrow cells.
Tr4 heterozygous mutation results in decreased erythroid cell proliferation without altering apoptosis. Colony-forming assays were performed by plating lineage negative bone marrow cells in erythroid MethoCult (M3334; see “Materials and methods”), imaged, (A) the number of erythroid colony-forming unit (CFU-E) colonies quantified after 3 days and (B) burst-forming unit (BFU-E) colonies after 14 days. (C,D) Bone marrow cells were isolated from WT and Tr4+/− femurs and stained with α-CD71, α-TER119, α-CD44 (as in Figure 2), and Annexin V antibodies (to assess apoptosis), and the total percentage of cells within each gate were quantified. (E,F) Bone marrow cells were stained with α-CD71, α-TER119, α-CD44, and α-Ki67 antibodies (to assess proliferation), and the total percentage of cells within each gate was quantified. (G) Stage III (CD71+TER119+) erythroid cells were stained with α-Ki67 and DAPI antibodies. Gating for stages of proliferation are indicated on the left and the percentage of cells within each gate is graphed on the right. *P ≤ .05 as determined by Student t test comparing Tr4 heterozygous mutant with WT bone marrow cells.
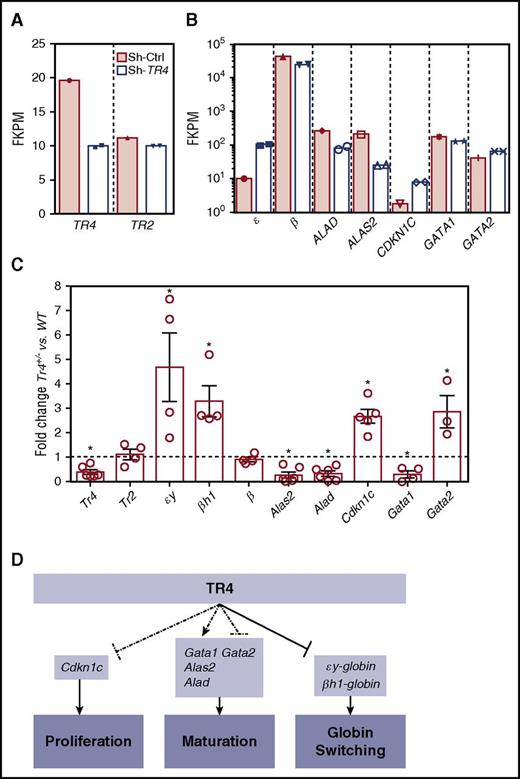
Expression of essential erythroid differentiation and proliferation genes are altered in Tr4+/− mutants
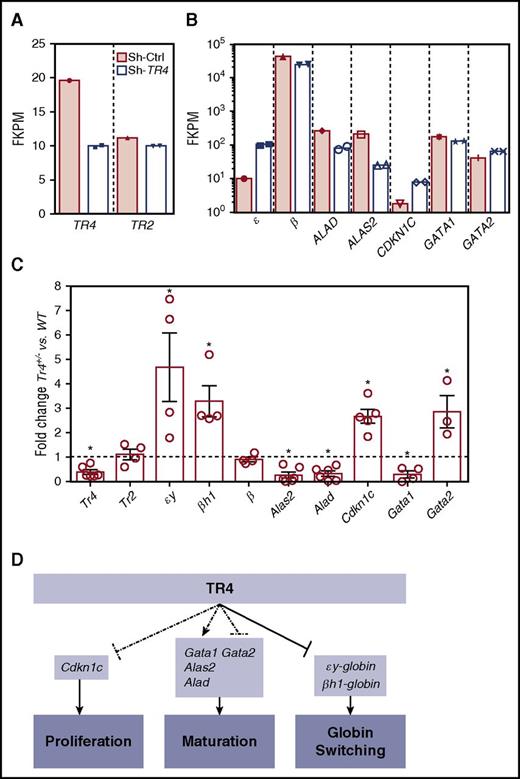
These experiments provide direct evidence that the orphan nuclear receptor TR4 functions in erythroid cells to regulate both differentiation and proliferation. To investigate the mechanisms that result in these observed deficiencies in erythroid cell physiology, we compared these results characterizing the murine mutants with previously published human RNA-seq data generated by our laboratory in which CD34+ cells were induced to undergo erythroid differentiation with or without infection with a lentivirus containing short hairpin RNA (shRNA) against TR4 (shRNA-TR4) or a scrambled control (shRNA-Ctrl, GSE5476).36 Differentially expressed genes (when compared with shRNA-Ctrl) whose function has been described in erythroid proliferation and differentiation were analyzed as candidate TR4 target genes.36 shRNA-TR4 in the human erythroid progenitor cells resulted in a 50% to 60% reduction in the abundance of TR4 transcripts (notably without altering TR2 expression; Figure 6A). As anticipated, one known TR4 direct target gene,4 human ε-globin, exhibited increased expression after TR4 knockdown, although there was no change observed in adult β-globin (β) transcription (Figure 6B). Two other genes that are known to be required for erythroid differentiation and heme biosynthesis, ALAD (reduced fourfold) and ALAS2 (reduced 3.6-fold), as well as 1 gene with a well-characterized role as an inhibitor of cellular proliferation, CDKN1C (increased fourfold), and the erythroid differentiation genes GATA1 (reduced approximately 1.5-fold) and GATA2 (increased 1.5-fold) were examined. These same transcripts were next examined in erythroid cells of Tr4+/− and WT mice (Figure 6C).
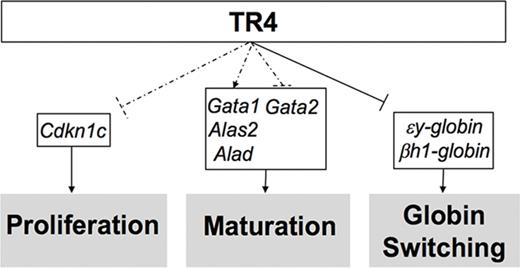
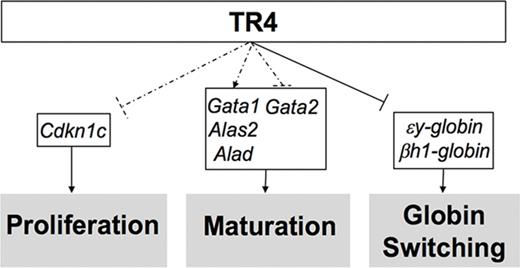
Altered expression of select erythroid differentiation and proliferation genes upon reduction of TR4 activity in human and murine erythroid cells. (A,B) RNA-seq analysis from CD34+ cells infected with a sh-TR4 lentivirus, as previously reported,36 and RNA-seq results from control jumbled shRNA (shaded bars) or the shRNA-TR4 (white bars) values are represented as fragments per kilobase per million reads (FKPM).36 (C) qRT-PCR quantification of multiple genes expressed in erythroid cells of stage III (CD71+TER119+) cells sorted from both WT and Tr4+/− mouse total bone marrow. The fold changes were calculated using ∆∆Ct with WT set to 1 (indicated by the dashed line). (D) Schematic summarizing the known functions of TR4 in murine erythroid cells, including these data. Dashed lines indicate correlative data indicating Cdkn1c, Gata1, Alas2, Alad, and Gata2 are TR4 candidate target genes regulating erythroid proliferation and maturation. Solid arrows from TR4 to εy-globin and βh1-globin indicate direct transcriptional regulation during globin switching. *P ≤ .05 as determined by Student t test comparing Tr4 heterozygous mutant with WT FACS-purified CD71+TER119+ cells.
Altered expression of select erythroid differentiation and proliferation genes upon reduction of TR4 activity in human and murine erythroid cells. (A,B) RNA-seq analysis from CD34+ cells infected with a sh-TR4 lentivirus, as previously reported,36 and RNA-seq results from control jumbled shRNA (shaded bars) or the shRNA-TR4 (white bars) values are represented as fragments per kilobase per million reads (FKPM).36 (C) qRT-PCR quantification of multiple genes expressed in erythroid cells of stage III (CD71+TER119+) cells sorted from both WT and Tr4+/− mouse total bone marrow. The fold changes were calculated using ∆∆Ct with WT set to 1 (indicated by the dashed line). (D) Schematic summarizing the known functions of TR4 in murine erythroid cells, including these data. Dashed lines indicate correlative data indicating Cdkn1c, Gata1, Alas2, Alad, and Gata2 are TR4 candidate target genes regulating erythroid proliferation and maturation. Solid arrows from TR4 to εy-globin and βh1-globin indicate direct transcriptional regulation during globin switching. *P ≤ .05 as determined by Student t test comparing Tr4 heterozygous mutant with WT FACS-purified CD71+TER119+ cells.
Flow-sorted CD71+TER119+ (stage III)32-34 cells were isolated for RNA extraction and subsequent qRT-PCR analysis to determine whether the candidate target genes identified from the human RNA-seq data are also differentially expressed in Tr4+/− mouse erythroid cells (Figure 6C). Consistent with the changes observed in human erythroid cell differentiation, CD71+TER119+ cells recovered from Tr4+/− mice displayed a significant increase in the expression of εy-globin and βh1-globin, but no changes were observed in the abundance of either Tr2 or β-major (Figure 6C). Candidate target genes that are critical for heme biosynthesis and erythroid differentiation, Alad and Alas2, were both significantly lower in the Tr4+/− mutants (Figure 6C). The expression of Cdkn1c was significantly increased, and could possibly be the mechanism by which TR4 modulates cell proliferation in erythroid cells. Additionally, Gata1 expression levels were diminished in Tr4+/− cells with a corresponding increase in Gata2 expression (Figure 6C).37,38 With the exception of εY-globin, which is known to be directly regulated by direct repeat erythroid-definitive,3,4 whether any of the other differentially expressed genes are direct or indirect targets of TR4 is still to be determined. These findings are summarized in schematic form in Figure 6D. Taken together, the ex vivo results from the analysis of human erythroid cell differentiation19 correspond closely to the results presented here on in vivo erythroid differentiation in the mouse, indicating that TR4 regulates differentiation and proliferation in mammalian erythroid cells.
Discussion
The activity of TR4 acting as one component of the first identified erythroid repressor complex is now well established.1,3,4,12,13,39 TR4 has been shown to bind to the human fetal γ-globin gene direct repeat promoter element, a sequence that is frequently mutated in hereditary persistence of fetal Hb syndromes leading to abnormally high levels of fetal γ-globin gene expression in adults.3,13 However, the identification of additional direct target genes within erythroid cells is incomplete. Here we report that Tr4−/− mutant mice, when examined in a congenic genetic background, expire between E7.0 and E9.5, and that Tr4+/− nonetheless exhibit defective erythroid differentiation and decreased proliferation, suggesting that TR4 plays a far more pervasive role in both embryonic development and erythropoiesis than previously suspected. Furthermore, Alad, Alas2, and Cdkn1c were identified as novel TR4 (direct or indirect) target genes in differentiating human and murine erythroid cells. Elucidation of TR4 functions during erythroid differentiation and its functional contribution to transcriptional hierarchies will further expand our understanding of erythroid cell development and provide insight into the potentially unanticipated consequences of targeting TR4-containing transcriptional complexes for possible therapeutic intervention in treating the β-globinopathies.
We were surprised to observe the early gestational embryonic lethality conferred after breeding Tr4−/− mice into genetic homogeneity because we previously reported that Tr4−/− mice in an outbred background were viable with no obvious hematological phenotype, whereas the compound loss of both Tr2 and Tr4 resulted in periimplantation lethality.3,13 The lethality observed in Tr2−/−:Tr4−/− embryos was attributed to growth and differentiation defects and increased apoptosis at approximately E7.5.40 Earlier, Liu et al reported embryonic lethality of a Tr4−/− mouse line when backcrossed into a congenic background; however, neither hematological nor developmental analyses were reported.16 Functional examination of TR2 and TR4 has been assessed in cultured embryonic stem cells, where TR4 was reported to play a role in stem cell self-renewal, differentiation, and proliferation specifically through the regulation of Nanog and direct binding to the promoter direct repeat 1 site of Oct-3/4.40 The present data provide direct evidence that the erroneously reported genetic redundancy and functional compensation between TR2 and TR4 that were observed in mixed lineage mice was almost certainly from the activities of genetic modifiers in outbred mice. Although it would be of interest to examine the phenotypes of the Tr4 mutation in other genetic backgrounds to identify the modifiers, such investigations are beyond the scope of the present studies.
The age at which Tr4−/− embryos begin to exhibit lethality precedes the initiation of erythroid cell development; we therefore sought to examine heterozygous animals for possible hematological phenotypes. In both Tr4+/− fetal livers and adult bone marrow cells, we documented defective erythroid differentiation, which is consistent with our previous study showing that conditional, simultaneous ex vivo deletion of Tr2 and Tr4 also affected erythroid differentiation and derepressed fetal globin.5 To identify the possible underlying cellular mechanisms that contributes to this phenotype, erythroid cells were analyzed for alterations in apoptosis, proliferation, and gene expression.
Previous studies implicated TR4 as a regulator of both apoptosis and proliferation.40-43 Retinoic acid–induced apoptosis has been reported to be mediated through TR4 in P19 mouse teratocarcinoma cells,42 which suggests that a reduction in TR4 abundance might lead to increased apoptosis in vivo. TR4 has also been shown to promote the proliferation of myeloid cells after its enforced expression by recombinant retroviral infection.41,44 In a mixed genetic background, Tr4−/− mice have decreased granule cell proliferation,43 and estrogen receptor–induced proliferation in the MCF-7 breast cancer cell line is reportedly inhibited through TR4 interaction with estrogen receptor.45 In summary, the present studies show that differentiation is impaired in Tr4+/− mice, and the cells exhibit decreased proliferation, which likely contributes to the observed reduction in differentiated erythroid cells.
To identify candidate erythroid TR4 target genes, we examined published RNA-seq data from erythroid differentiated human CD34+ cells infected with lentiviral-shRNA-TR4.36 Those studies revealed that there was significant alteration in the expression of erythroid differentiation and proliferation genes. In the experiments reported here, we identify several candidate target genes that regulate erythroid differentiation and proliferation in the mouse and confirm their cross-species concordance by quantifying the expression of the human-homologous murine genes in flow-sorted erythroid cells. Whether these validated candidates are direct or indirect targets of TR4 is yet to be determined. However, TR4 chromatin immunoprecipitation–seq datasets from differentiated human CD34+ cells36 displayed no obvious binding peaks, suggesting that they could be indirect targets (data not shown).
Two of the differentially regulated target genes, Alas2 and Alad, encode enzymes in the heme biosynthetic pathway, and are both vital for erythroid differentiation.26,46 ALAS2 is the rate-limiting enzyme in the heme biosynthesis pathway and catalyzes the condensation of glycine and succinyl-coenzyme A to 5-aminolevulinic acid in mitochondria.47-49 Alas2 null mutant mice display decreased erythroid differentiation, aberrant iron accumulation, and increased oxidative stress.46 Furthermore, a conserved regulatory region within intron 8 of Alas2, bound by Gata1, is required for its tissue-specific expression during erythroid differentiation.25 In humans, mutations in either ALAS2 or GATA1 can lead to X-linked sideroblastic anemia.36,50-52 Our data also reveal diminished Gata1 expression in Tr4+/− animals, both in human CD34+ cells treated with lentiviral shRNA-TR436 and in murine Tr4+/− CD71+TER119+ erythroid cells, with correspondingly increased expression of Gata2. These data support the observed decreases in the number of differentiated erythroid cells because induced Gata1 expression is required for erythroid differentiation.37,38
Another candidate target gene that is differentially regulated in Tr4+/− mice vs WT mice, Alad,53 is the second enzyme in the heme biosynthesis pathway and fuses 2 molecules of δ-aminolevulinate to generate porphobilinogen.47 Heme is an essential component of Hb used to bind iron and is thought to function in translation, transcription, and protein degradation during erythroid differentiation.47 Additionally, when Alad is inhibited by binding to lead,54,55 the lifespan of circulating red blood cells is reduced.55 Thus it seems probable that TR4 acts somewhere upstream of both heme biosynthetic pathway genes to control Alas2 and Alad transcription in erythroid cells, thereby promoting coordinately regulated heme accumulation and cell differentiation.
Cdkn1c, also known as p57, is a cell-cycle inhibitor belonging to the Cip/Kip family, which acts on the cyclin-dependent kinases Cdk2 and Cdk4 to negatively regulate cell-cycle progression.56 In hematopoietic stem cells, Cdkn1c is highly expressed and required to maintain a quiescent state.57 We observed an increase in Cdkn1c expression in Tr4+/− erythroid cells along with a coincident decrease in cellular proliferation, suggesting that TR4 directly or indirectly represses Cdkn1c expression.
In summary, these data provide evidence that TR4 does not act in a redundant manner with TR2, as previously reported.5,15,18 We conclude that TR4 is required for the normal differentiation and proliferation of erythroid cells, in addition to its previously characterized roles in embryonic and fetal globin gene repression.3,4,39 Given the early embryonic lethality of Tr4−/− embryos, it is also apparent that TR4 is required before the initiation of erythropoiesis. Additional studies will be required to identify the detailed mechanisms by which TR4 regulates the observed changes in erythropoiesis and early embryogenesis. Additional outstanding questions also remain, such as whether TR4 functions as a homodimer, as a heterodimer with TR2,1 or as a partner of other nuclear receptors.36 Better definition of the mechanistic underpinnings of TR4 involvement in erythroid development will both advance our understanding of erythroid differentiation and possibly aid in the development of safer and more effective therapeutics to treat β-globinopathies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank numerous colleagues for provocative questions and constantly stimulating interactions over the years and are grateful for the expert comments of N. Mohandas (New York Blood Center) in previewing this manuscript.
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (grant F32DK108493) (M.P.L.) and National Heart, Lung, and Blood Institute (grant U01HL117658) (J.D.E.). N.J. received support as an Excellence in Hemoglobinopathies Research Award Translational Research Scholar (grant U01HL117658).
Authorship
Contribution: M.P.L. designed, performed, and analyzed experiments and wrote the paper; O.T. designed and performed experiments, L.S. performed and analyzed experiments, N.J. analyzed experiments and wrote the paper; D.L. commented on and analyzed experiments; and J.D.E. designed and analyzed experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for L.S. is the State Key Laboratory of Experimental Hematology, Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin, China.
Correspondence: James Douglas Engel, Department of Cell and Developmental Biology, University of Michigan Medical School, 3035 BSRB, 109 Zina Pitcher Place, Ann Arbor, MI, 48109-2200; e-mail: engel@umich.edu.