Abstract
As individuals with germ line predisposition to hematologic malignancies are diagnosed with increasing frequency, the need for clinical surveillance has become apparent. Unfortunately, few prospective data are available, so recommendations are based on collective experience and expert consensus. There is general agreement to advocate for expert consultation or referral of patients to centers with expertise in these syndromes, since presentations and disease progression can be subtle, and treatment strategies must be tailored. Here, we summarize and integrate expert consensus recommendations and medical management considerations for the patient newly diagnosed with a leukemia predisposition disorder. Indications to consider additional studies and referral for allogeneic stem cell transplantation are also discussed.
Introduction
With the inclusion of germ line predisposition to myeloid malignancies for the first time within the most recent leukemia classification scheme,1 the World Health Organization has designated familial hematopoietic malignancies as an essential component of leukemia diagnosis (Table 1). With the increasing use of molecular profiling and the emergence of panel-based testing specifically for the detection of germ line syndromes that predispose to hematopoietic malignancies,2-6 increasing numbers of patients and families will be recognized as having an inherited cancer predisposition syndrome. Typically, the first member of the family to be identified has already developed a hematologic malignancy or cytopenias, but testing of additional family members results in the identification of those who have the deleterious mutation but who have not yet been diagnosed with cancer. In this review, we will summarize surveillance recommendations from expert panels and from our own experience for patients diagnosed genetically and/or clinically as well as special considerations for the treatment of malignancies that develop in these individuals.
Consensus guidelines for leukemia predisposition syndromes
In response to the paucity of data to inform optimal surveillance strategies, expert panels have convened to summarize consensus opinions for some of the leukemia predisposition syndromes (Table 2), including Fanconi anemia,7 Diamond-Blackfan anemia,8 Shwachman-Diamond syndrome,9 and dyskeratosis congenita/telomere biology disorders.10
Because these sets of guidelines are available, we will not discuss these specific disorders in depth in this review, but we will highlight some key recommendations and general considerations. Surveillance considerations vary depending on the potential benefit of early treatment prior to the development of myeloid or lymphoid leukemia and the likely utility of monitoring strategies. Leukemia outcomes with these disorders may be affected by additional organ system comorbidities, risk of toxicities from intensive leukemia-directed therapy, risk of BMF resulting in prolonged or intractable cytopenias after leukemia-directed therapy, and risk of relapse or refractory disease. Consultation with a medical expert in BMF or cancer predisposition is recommended to develop personalized surveillance strategies in this rapidly advancing field.
Recommendations based on expert opinion
For the first time this year, the WHO has included germ line predisposition to myeloid malignancies in its revised classification scheme (Table 1).1 In addition to the aforementioned BMF syndromes described above, additional myeloid leukemia predisposition disorders include germ line mutations in ANKRD26, CEBPA, DDX41, ELANE, ETV6, GATA2, HAX1, RUNX1, SAMD9, SAMD9L, and SRP72.11-20 Inherited predisposition to lymphoid malignancies has also been described, including germ line ETV6, PAX5, and TP53 mutations.18,19,19,21-29 Familial IKZF1 mutations are also likely to emerge as a distinct syndrome (Table 2).30
Most of the recommendations that follow for inherited leukemia syndromes are based on expert opinion and experience given the paucity of prospective data. Therefore, although guidelines are emerging, it is important to recognize the need to individualize recommendations for each case depending on the specific disorder, personal/family history, standards within particular practice norms (eg, pediatric versus adult oncology approaches), local preferences, and financial constraints. Moreover, we welcome future refinement of these recommendations as expanded knowledge of these syndromes emerges and as we perform prospective clinical monitoring of at-risk individuals.
These surveillance recommendations apply to people found to have known deleterious or likely deleterious variants or who meet clinical diagnostic criteria for a cancer predisposition disorder yet remain genetically undefined. Individuals with variants of uncertain significance (VUS) are encouraged to participate in research studies that explore the segregation of VUSs with malignancies so that new associations can be established. Generally, clinical testing of relatives is not recommended for a VUS, though further evaluation in the research setting may be helpful. Demonstration of cosegregation of a variant with affected individuals within families may facilitate pathogenic classification of a variant. When possible, genetic testing within a family should be sent to the same testing laboratory for consistency in methodology and analysis. An important caveat regarding the classification of variants has to be highlighted, however. Inconsistencies exist regarding the distinctions between particular variants and designations that ascribe disease causality: benign vs VUS vs likely/known deleterious.31 Therefore, different laboratories may classify variants differently, leading to tremendous confusion for physicians, genetic counselors, and family members, especially when family members obtain clinical testing through different laboratories. The use of variant reporting registries such as ClinVar and ClinGen may bring more consistency through standardization of variant evaluation and centralized assessment of phenotypic associations.32
Recommendations for baseline assessments for the patient newly diagnosed with a genetic predisposition to leukemia
For a discussion of screening and diagnostic evaluation for a genetic predisposition disorder, the reader is referred to other recent papers and reviews.6,11,33-35 Once an individual is first diagnosed with a leukemia predisposition syndrome (Table 3), experts and consensus panels have generally recommended consultation with an expertise in this area, genetic counseling, baseline CBC with differential, and bone marrow aspirate and biopsy, including cytogenetic and molecular testing.
Consultation with an expertise in this area
This is recommended given the highly specialized and rapidly advancing evaluations and tailored treatments for these patients, as discussed below. If travel to a center of excellence is not possible, then telephone discussion with an expert consultant may be helpful. Specific medical comorbidities particular to the specific syndrome and/or clinical state of the individual or family history can be assessed and a management plan developed. During consultation, a “family letter” or summary can be given to the affected individual that outlines the variant found, describes the underlying inherited syndrome and common clinical implications, and provides cancer prevention strategies and contact information for future counseling and clinical testing. This type of written correspondence is useful to the individual to provide a record of the discussion and can be shared with others in the family as well as the patient’s primary physicians to disseminate the information provided.
Genetic counseling
A central component of follow-up or referral to a center of excellence is the opportunity for an affected individual to meet with a genetic counselor trained in germ line syndromes that predispose to hematopoietic malignancies. Detailed family histories may be obtained by genetic counselors knowledgeable in these syndromes. Collection of prior medical records is recommended to confirm leukemia diagnoses and associated clinical manifestations such as treatment-related toxicities or comorbidities. Counseling generally includes a discussion about genetic inheritance patterns, clinical manifestations of the detected variant, implications for other family members, and a discussion of testing options for others within the family. Often genetic counselors are available to provide basic background that corrects patients’ misunderstandings. Genetic counselors can also help navigate insurance coverage for consultation and surveillance, which can be difficult since many insurance companies have not yet established policies that recognize the clinical importance of genetic testing for germ line susceptibility syndromes for the hematologic malignancies, the importance of cancer surveillance, or the need for individualized tailored therapies, leading to delay or even denial of surveillance which in turn may adversely affect clinical outcomes.
Genetic counseling should be offered to family members of the proband. Siblings of an affected proband are at risk of having the disorder even if clinically asymptomatic, since the phenotypic spectrum of these disorders is highly variable.2-4,12,36,37 Surveillance should be discussed and offered to affected family members per recommendations for the specific syndrome.
Baseline CBC with differential
Patients may present with cytopenias that range in severity from mild subclinical cytopenias to severe aplastic anemia. The hematologist should directly examine the blood smear for dysplasias or other clues suggesting marrow pathology such as nucleated red cells and teardrop cells. Attention should be paid to each of the three blood cell lineages: the total white blood cell count and differential to look for the presence of early myeloid cells or blasts that could signal the existence of a myelodysplastic or overt neoplastic process; the red blood cell count/hemoglobin level, with attention to the mean corpuscular volume, the elevation of which can indicate dysplasia or BMF; and the platelet count, which can be at the low end of normal range or below the normal range in germ line RUNX1, ETV6, and ANKRD26 mutation carriers. Platelet aggregation studies may be abnormal with RUNX1, ETV6, or ANKRD26 disorders but are complicated by the low platelet numbers at baseline in these individuals.
Bone marrow aspirate and biopsy, including cytogenetic and molecular testing
A baseline bone marrow biopsy when a leukemia predisposition disorder is first identified in an adult is important to rule out the existence of a bone marrow malignancy, assess for BMF, and for future comparative use. This is especially important when cytopenias and/or macrocytosis are seen on the screening CBC. Some hematologists do not perform baseline bone marrow examinations in the absence of CBC abnormalities, and additional data are needed to inform the optimal timing of bone marrow examinations. Consideration should be given to the particular family history of potential for disease anticipation within the family, which could prompt a bone marrow assessment in the absence of abnormal CBC values. Disease anticipation refers to manifestation of disease at progressively earlier ages in subsequent generations and is a common occurrence in some familial hematopoietic malignancies.38-42 In the absence of clinical urgency, the timing of the initial bone marrow examination is often deferred for infants or very small patients for whom marrow examinations are technically more challenging and anesthesia poses higher risks.
If a bone marrow examination is performed, both core biopsy and aspirate samples should be obtained. The core biopsy yields important information regarding overall bone marrow cellularity and fibrosis, and the aspirate allows a detailed morphologic assessment of dysplasia and the most accurate enumeration of the blast percentage. Baseline cytogenetic and FISH studies are also recommended. Occasionally the presence of a hematologic malignancy may be flagged by the presence of a characteristic chromosomal translocation and/or gene mutation, including for example NPM1, FLT3, or CEBPA. Somatic mutation panels can provide useful information regarding clonal evolution but should be considered in the context of the rest of the blood and marrow evaluation. While somatic mutation analysis of patients with MDS or AML informs risk stratification and treatment decisions, further study is needed to understand the clinical significance of somatic mutations noted in the absence of MDS or AML.
Recommendations for clinical follow-up
Follow-up and surveillance should be tailored to the specific predisposition syndrome in accordance with available data, expert opinion, or consensus recommendations for that disorder. Regular follow-up facilitates the coordination of multidisciplinary care with other relevant subspecialties, updating of the family history, and affords access to the latest advances in the management and treatment of these disorders. Ongoing education should be provided about any indicated precautions for patients with cytopenias, as well as discussion of signs and symptoms of leukemia. Signs and symptoms that should prompt a patient/family to seek medical evaluation include recurrent or severe fevers, excessive or persistent fatigue, unexplained weight loss, excessive bruising/bleeding, lymphadenopathy, and persistent or progressive bone pain. Patients and families may also appreciate learning about opportunities for participation in research to advance knowledge about these disorders.
Regular clinical evaluations are recommended to assess for clinical signs or symptoms of malignancy as well as physical examinations for lymphadenopathy, hepatosplenomegaly, or other clinical findings suggestive of leukemia. Opinion varies as to the recommended routine frequency of such clinical evaluations, but a frequency between 6 and 12 months is generally agreed to be reasonable depending on the clinical status of the patient and the preferences of the physician and patient. The frequency of medical examinations may be increased as clinically indicated.
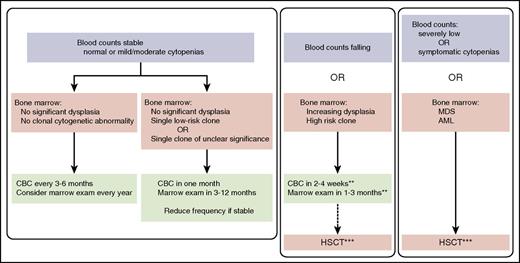
As discussed above for the frequency of clinic visits, the frequency of routine peripheral blood count screening is debatable. However for predisposition to MDS or BMF syndromes, regular blood count surveillance every 3 to 6 months is recommended (Figure 1). A persistent or progressive drop in blood counts warrants a careful medical history and examination to assess for potential causes, most commonly due to medications or infections. Such secondary cytopenias are typically transient, so blood counts should be followed to ascertain recovery to baseline within a reasonable time (typically 2-4 weeks) after resolution of the inciting infection or discontinuation of the offending medication. Persistently low or falling blood counts well after resolution of the inciting event raise concern for an evolving bone marrow disease and therefore warrant further evaluation, including a bone marrow examination. In some cases, rising blood counts without an apparent cause may also herald MDS.
Surveillance for predisposition to MDS/AML. A general approach to surveillance is outlined. A discussion of low-risk clones is provided in the text. HSCT, hematopoietic stem cell transplant. *Excludes baseline dysplasia typically associated with specific disorders. **See text for additional discussion. ***For some disorders, chemotherapy may be considered to treat leukemia.
Surveillance for predisposition to MDS/AML. A general approach to surveillance is outlined. A discussion of low-risk clones is provided in the text. HSCT, hematopoietic stem cell transplant. *Excludes baseline dysplasia typically associated with specific disorders. **See text for additional discussion. ***For some disorders, chemotherapy may be considered to treat leukemia.
Data are scarce to inform the benefit of routine screening of blood counts for other acute leukemia predisposition disorders, particularly those predisposing to acute lymphoid malignancies where leukemia prognosis is generally good. A prospective nonrandomized observational study of patients with germ line TP53 mutations led by investigators in Toronto compared outcomes with or without cancer surveillance including blood counts every 3 to 4 months and demonstrated improved survival in the surveillance group. Most of the malignancies were solid tumors, and numbers were too small to draw specific conclusions regarding the effect of surveillance on leukemia incidence and outcomes in this patient population.43 However, early detection of cytopenias or peripheral blasts prior to the development of clinical symptoms, such as fevers or infections, may avoid complications that impact outcomes. Certainly vigilance for potential signs of hematologic malignancy in the clinical history, examination, or other laboratory tests and prompt examination of the peripheral blood cell counts and consideration of a bone marrow examination are warranted.
Progression to AML may be gradual, but abrupt presentations are also possible. Early signs of clonal evolution may be apparent in the marrow prior to the onset of leukemia. Blood counts alone are not necessarily a sensitive marker, and cytopenias may not be apparent until after leukemia has developed. The authors have personal experience with patients who developed MDS noted on routine surveillance marrow examination without changes in the baseline blood counts. Dropping blood counts are often late signs of leukemia, so the strategy of waiting until the patient appears sick may miss the window of opportunity for treatment when outcomes are best prior to leukemia onset. The benefit of marrow surveillance must be weighed against the risks of the procedure, particularly when general anesthesia is required for young children. A frank discussion to engage patient/family input regarding surveillance is essential.
Regular bone marrow surveillance examinations include aspirate morphology, cytogenetic analysis/FISH, and molecular studies. For BMF disorders in which the marrow cellularity is decreased and metaphase cell numbers may be low and subject to sampling biases, screening of large numbers of interphase cells by FISH increases the detection of clonal populations. Increasing marrow dysplasia noted on surveillance examinations also warrants closer surveillance (Figure 1). Nonmalignant causes of marrow dysplasia, such as deficiencies of vitamin B12 or folate, medications, or infections, should be evaluated. We emphasize that many leukemia predisposition disorders are associated with baseline dysplasia that is not associated with impending leukemia. For example, hypolobation and hypogranulation of myeloid cells, irregular nuclear morphology of erythroid cells, and small hyponucleated megakaryocytes are frequently seen at baseline in patients with Fanconi anemia, Shwachman-Diamond syndrome, or germ line ANKRD26 mutations. Micromegakaryocytes and splaying of the megakaryocyte nuclei are frequent findings in GATA2 disorders.44 For this reason, having a baseline bone marrow biopsy/aspiration can be critical to allow comparison that would afford assessment of change in baseline, which would signal disease progression.
Single clonal cytogenetic abnormalities of unclear clinical significance may also warrant increased frequency of surveillance visits to assess for progression to MDS, particularly for clonal abnormalities associated with a high risk of progression to leukemia, such as monosomy 7. Amplification of 3q26q29 has been associated with a high risk of progression to leukemia in some patients with Fanconi anemia.45 Some cytogenetic clonal abnormalities commonly arise in some disorders but are not associated with impending leukemia when present as an isolated abnormality. For example, isochromosome 7 or del20q frequently arise and may persist for many years in patients with Shwachman-Diamond syndrome without progression to leukemia.46 Interestingly, the breakpoint for del20q is typically proximal to the EIF6 gene leading to loss of 1 copy of EIF6. Since SBDS functions in promoting the release of EIF6 to allow joining of the 40S and 60S ribosomal subunits,47 it has been proposed that haploinsufficiency of EIF6 might compensate for SBDS deficiency.48 The acquisition of clones bearing multiple cytogenetic abnormalities within the same cell is an ominous finding that may portend the development of leukemia.
Decreasing bone marrow cellularity often raises concern for progressive marrow disease. However, marrow cellularity is patchy and subject to sampling variation, and therefore, cellularity should be considered in the context of peripheral blood counts. In some cases, increasing marrow cellularity may be a harbinger of evolving MDS. An integrated assessment of the marrow and blood is recommended (Figure 1). For example, a cytogenetic clonal abnormality arising in the context of increasing marrow dysplasia and falling or low blood counts raises more concern than an isolated clonal abnormality of unclear clinical significance. Concerning findings in the marrow may be followed by more frequent blood counts, typically at least once a month, and a follow-up marrow examination in 1 to 6 months, depending on the degree of concern, to assess for progression. If the marrow improves or if there are no signs of worsening abnormalities, the frequency of blood counts and marrow examinations may be reduced back to baseline surveillance.
For patients with predisposition to acute leukemias without MDS, particularly the lymphoid leukemias, the benefit of routine surveillance with serial bone marrow examinations is less clear. Outcomes for acute lymphoid leukemias in children are generally excellent with current nontransplant treatments for acute lymphoid malignancies. In these patients, lymphoid malignancies often develop quickly without the prolonged preleukemic phase often seen with myeloid leukemia predisposition syndromes. Examination of the bone marrow is indicated if clinical suspicion is raised by the medical history, examination, or blood counts.
Testing for somatic mutations acquired in the bone marrow is now widely clinically available. For example, acquisition of a variety of somatic mutations in genes including CSF3R is observed in patients with severe congenital neutropenia who have progressed to AML.49-52 However, these same CSF3R mutations have been observed in a few patients with severe congenital neutropenia without progression to leukemia over several years of follow-up.53 Similarly, mutations associated with MDS/AML have been observed without leukemia in young patients with constitutional RUNX1 mutation.54 Somatic mutation analysis may be useful to guide the frequency of marrow evaluations. However, we stress that for the most part, the clinical implications of somatic mutations in patients with cancer predisposition remain to be ascertained.
Treatment
The curative therapy of choice for MDS or AML for many of the leukemia predisposition disorders is an allogeneic HSCT. Because outcomes of myeloid leukemias in these patients are often poor for many of these disorders due to high treatment-related toxicities and high risk of relapse or refractory disease, HSCT is best initiated prior to the development of leukemia. Although prolonged remissions have been reported with chemotherapy alone to treat leukemia for some disorders such as germ line CEBPA variants, the risk of leukemia recurrence is high due to the development of new independent leukemia clones.55 Discussion with an expert should include consideration of the specific leukemia-predisposition disorder, patient age, likelihood of cure, treatment-related risks, comorbidities, and donor availability. Timely HSCT prior to accumulation of a high blast burden may avoid the need for remission induction with intensive AML therapies that increase the risk of infection and organ toxicities. Early partnership with a transplant expert allows coordinated evaluation and follow up to facilitate timely initiation of transplant. Some of the leukemia predisposition disorders are associated with an increased risk of BMF causing prolonged or intractable pancytopenias following chemotherapy or radiation. For such patients, identification of an allogeneic stem cell donor prior to or at the time of commencement of AML-directed therapies is essential, since initiation of transplant may be required if recovery from myelosuppression is significantly delayed. In addition, some of the leukemia predisposition syndromes are associated with increased toxicities using standard doses of pretransplant AML-directed therapies. However, the risk of such treatment-related toxicities must be balanced against the potentially increased risk of refractory or relapsed disease with reduced intensity regimens.
Indications for HSCT include severe or symptomatic cytopenias, severe marrow dysplasia (particularly in the context of falling blood counts), complex or high-risk (eg: monosomy 7) cytogenetic abnormalities (particularly if the clones are large or increasing in size), and increasing blasts >5%. Bone marrow morphologic criteria to diagnose MDS for some of these disorders associated with baseline dysplasia remains a matter for debate, so consultation with a hematopathologist experienced with these disorders is recommended. For disorders associated with poor leukemia outcomes, the observation of marrow abnormalities suggestive of MDS warrants consideration of treatment, even if blood counts have not acutely changed from the patient’s baseline. Multiple abnormalities of the blood and marrow increase clinical concern (Figure 1). For many of the genetic or familial MDS/BMF disorders, initiation of transplant prior to the development of excess blasts or frank leukemia may reduce the risk of relapse and may avoid the necessity of pretransplant chemotherapy with its attendant toxicities. Nonetheless, preemptive transplant in the absence of any signs of clonal progression is not recommended at the present time, since only a subset of affected patients might progress to leukemia and some patients would be needlessly exposed to transplant risks. Research is ongoing to identify which patients might benefit from preemptive transplant prior to any signs of clonal evolution. For some of the marrow failure syndromes, the role of pretransplant chemotherapy to induce remission prior to transplant remains unclear due to the associated high morbidity and risk of treatment-related mortality.
Considerations for related family donors
Genetic counseling should be offered to family members. Healthy family members diagnosed with a leukemia predisposition syndrome should be counseled regarding cancer surveillance. If the genetic diagnosis is known, potential related donors should be tested for the mutation regardless of clinical findings, since the phenotypic spectrum is broad even between affected members within the same family. Clinical phenotypes of leukemia predisposition disorders are variable and may lack apparent physical findings. Many of the syndromic leukemia predisposition disorders are now recognized to also present without classical stigmata.2 Many patients with Fanconi anemia36 or Diamond-Blackfan anemia8 lack classical findings. A subset of patients with Shwachman-Diamond syndrome do not present with steatorrhea or neutropenia.56 The majority of patients with telomeropathies lacks the classical triad of leukoplakia, rash, and nail dystrophies.57 GATA2 spectrum disorders may be indistinguishable from de novo MDS.58 Siblings of a proband with an inherited predisposition to MDS/AML are at risk for the same disorder and should be evaluated prior to serving as a marrow donor to avoid inadvertently selecting an affected family member.
Counseling of family members and/or their legal guardians should be provided at the time of genetic testing and upon return of results. Savings of cost and time may be garnered by site-specific testing of the mutation(s) identified in the proband. For patients with Fanconi anemia, siblings may be tested by chromosomal breakage testing. It has been proposed that consideration should be given to chromosomal testing of fibroblasts for potential family donors to avoid possible false negative results from testing blood due to lymphocyte somatic mosaicism.7 Similarly, family members of patients with short telomeres suspicious for an underlying telomeropathy should also be tested.
In situations in which there is clinical suspicion for a possible inherited disorder but genetic diagnosis is lacking, potential related donors should undergo a detailed medical evaluation with a focus on potential clues to an underlying genetic leukemia predisposition disorder. Attention should be paid to physical anomalies or medical history suspicious for the leukemia predisposition syndromes. Suspicion should be high if blood counts are even mildly depressed below a threshold neutrophil count of 1500/μL, platelet count <150 000/μL, a hemoglobin below the lower limit for age and gender, or if the mean corpuscular volume is elevated. Due to the broad phenotypic variation of these disorders, a bone marrow examination, including aspirate, biopsy, cytogenetic analysis/FISH studies, and molecular studies, is recommended even if the examination and blood count results are normal when a possible genetic or familial BMF/leukemia predisposition disorder is suspected.12,56,59 If family members have abnormal blood counts or abnormal marrow examination results, consideration should be given to unrelated donor options. If no abnormalities are found, the risks and benefits of using a seemingly healthy HLA-matched family donor for whom a genetic disorder cannot be completed ruled out vs unrelated donor options should be discussed with the patient and family. Early referral to a transplant specialist with expertise in these syndromes allows a team approach to patient care and monitoring. HLA typing and testing of siblings informs donor options, which in turn may guide the future timing of a donor search if clinically indicated. Patients known to have a rare HLA type may benefit from early activation of a donor search if worrisome findings arise during surveillance.
Long-term follow-up for germ line mutation carriers
Although HSCT is curative of the hematologic, and in some cases immunologic (eg: GATA2-associated disorders), manifestations of the leukemia predisposition disorders, many of these disorders are associated with additional nonhematologic complications that are not relieved by an allogeneic HSCT. These comorbidities require monitoring and treatment. For example, many of the BMF syndromes are associated with a high risk of endocrine disorders and congenital anomalies involving the skeletal system, heart, gastrointestinal tract, lungs, and central nervous system. In addition, some of these disorders also carry a high risk of solid tumors, such as squamous cell carcinomas of the head, neck, gastrointestinal tract, and vulva in Fanconi anemia and telomere biology disorders. Vaccination against human papillomavirus is recommended. Surveillance for solid tumors increases the opportunity to detect malignancies at an early stage when they are small and resectable for curative therapy. Prognosis of metastatic solid tumors is generally poor. As increasing numbers of pediatric patients are surviving into adulthood, transition strategies from pediatric to adult medical care. Identification of adult hematologists with expertise in these disorders is urgently needed.
Conclusion
In sum, our goal here has been to integrate and summarize data and expert recommendations for the clinical assessment and management of patients with a germ line genetic risk for hematopoietic malignancies. These recommendations are based on collective personal experience and expert consensus opinion in caring for these individuals and families, and there is an urgent need for further study to inform management. Until then, the utility of these recommendations will continue to be assessed and revisions will continue to evolve based on additional experience. Enrollment of affected individuals and family members in research protocols and registry studies is encouraged whenever possible so that we may continue to learn from their experience.
Acknowledgments
The authors thank their patients and families for the inspiration, which has advanced the field of inherited hematopoietic malignancies.
This work was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant R24 DK099808 (A.S.).
Authorship
Contribution: L.A.G. and A.S. wrote the manuscript and constructed the tables and figure.
Conflict-of-interest disclosure: L.A.G. receives royalties from a coauthored article on inherited hematopoietic malignancies in UpToDate. A.S. declares no competing financial interests.
Correspondence: Akiko Shimamura, 300 Longwood Ave, Karp 8210, Boston, MA 02115; e-mail: akiko.shimamura@childrens.harvard.edu.