Abstract
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare, aggressive hematologic malignancy. Data on the biology of BPDCN is limited, patient outcomes have historically been poor and there is no established standard of care. Nearly 100% of patients with BPDCN overexpress CD123, and considering the unmet medical need for these patients, targeting CD123 emerged as an attractive therapeutic target given its accessibility (cell surface) and differential expression.
UCART123 product candidate is based on genetically modified allogeneic T-cells (derived from healthy donors, so-called "off the shelf") containing an anti-CD123 CAR (CD123 scFv-41BB-CD3z) and a RQR8 depletion ligand that confers susceptibility to rituximab. To minimize the risk of graft-versus-host disease, the expression of the T-cell receptor (TCR) is abrogated through the inactivation of the TCR a constant (TRAC) gene, using Cellectis' TALEN® gene-editing technology.
The specific in vitro anti-tumor activity of UCART123 cells against an established BPDCN cell line (CAL-1) or CD123+ primary BPDCN samples was demonstrated using several assays. The cytotoxicity assay showed that UCART123 cells eliminate CAL-1 cells (65.6±2.7%) at low E:T ratios (1:1) and induced specific lysis of all BPDCN samples (from 15.7% to 77.9%). This cytotoxic activity was confirmed by T-cell degranulation and the secretion of IFNγ and other cytokines (IL2, IL5, IL6, IL-13 and TNF-α) by UCART123 cells when cultured in the presence of BPDCN cells.
To evaluate in vivo anti-tumor activity of UCART123 cells, we established three patient-derived xenografts (PDX1-3) from patients with relapsed BPDCN in NSG-SGM3 (NSG-S) mice. Upon engraftment, mice were randomized into five groups; and received either a single tail vein injection of vehicle, 10×106 TCRαβ KO control T-cells, or UCART123 cells (1×106, 3×106 or 10×106 cells). In PDX-1 model, all mice in vehicle-treated group died by D53, with high tumor burden in peripheral blood (PB, 30-65% engraftment), spleen and bone marrow (BM). UCART123 cells were detected in spleen (16.4% CAR+ cells) and BM (1.1% CAR+ cells) using human CD5 antibody or CD123-Fc protein, in a mouse injected with 10×106 UCART123 at D57. Six out of 9 (67%) mice in 10×106 UCART123 were alive and disease-free at the end of the study (D299), with remaining 3 mice dying without evidence of BPDCN (Days 155-280).
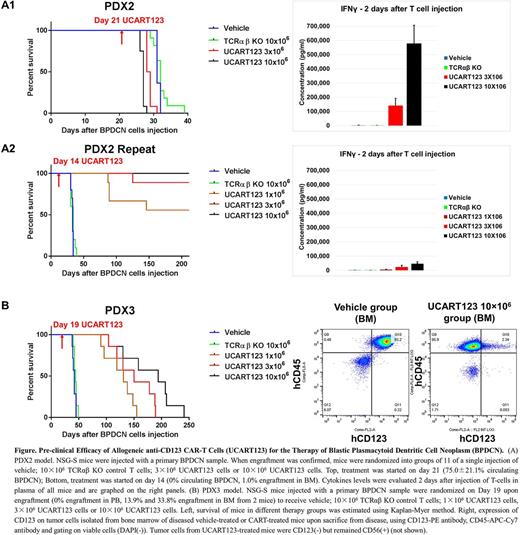
In PDX-2 model, UCART123 cells were injected upon confirmation of high tumor burden (75.0±21.1% engraftment in PB at D21). On D28, all the mice in 10×106 UCART123 group died. A high level of IFNγ was detected in PB from mice 2 days after treatment with UCART123 (Fig. A1, right panel). On D31-32, mice in vehicle group died with tumor burden approaching 100%, indicating extremely aggressive disease. Cytokine storm and/or extremely high tumor burden may have contributed to the demise of UCART123 treated mice. We repeated this in vivo PDX-2 study and started treatment at an earlier time point (D14, when engraftment reached 1% in BM). On D34, all mice in vehicle group were sacrificed due to tumor progression. In contrast, all mice in 10×106 UCART123; 8 out of 9 mice in 3×106 UCART123; and 5 out of 9 mice in 1×106 UCART123 were still alive at the end of the study (Day 210), without evidence of detectable BPDCN by PB flow cytometry (Fig. A2).
In PDX-3 model, 49 days after primary BPDCN sample injection, all the mice in vehicle were sacrificed due to high tumor burden in spleen and BM. In this model, we observed the emergence of CD123- BPDCN cells in all groups of UCART123-treated mice, causing relapse and early death after initial response (Fig. B). Mechanisms of CD123 loss are under investigation.
In summary, UCART123 causes specific killing of BPDCN cells, associated with antigen-specific T-cell degranulation and robust levels of IFNg production. Our preliminary data indicate long-term persistence of UCART123 cells in vivo in an NSG-S model of primary BPDCN, and confirm expected risk of deadly cytokine release syndrome at high tumor burden. Most importantly, UCART123 therapy results in BPDCN eradication and long-term disease-free survival in a subset of primary BPDCN PDX models. However, loss of CD123 expression may be anticipated and requires further studies. These results demonstrate pre-clinical proof-of principle of high anti-BPDCN activity of allogeneic UCART123 cells. A phase I trial of UCART123 in BPDCN is opened for enrollment (NCT03203369).
Galetto: Cellectis SA: Employment. Gouble: Cellectis SA: Employment. Smith: Cellectis: Employment. Neelapu: Karus: Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Poseida Therapeutics, Inc: Research Funding; Cellectis Inc.: Research Funding. Lane: Stemline Therapeutics: Research Funding; N-of-one: Consultancy. Guzman: Cellectis: Research Funding. Kantarjian: Amgen: Research Funding; ARIAD: Research Funding; Pfizer: Research Funding; Delta-Fly Pharma: Research Funding; Bristol-Meyers Squibb: Research Funding; Novartis: Research Funding. Pemmaraju: Stemline: Consultancy, Honoraria, Research Funding; LFB: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria; Cellectis: Research Funding; Novartis: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.