Abstract
There is a need to improve outcomes for patients with recurrent and/or refractory hematological malignancies. Immunotherapy holds the promise to meet this need, because it does not rely on the cytotoxic mechanism of conventional therapies. Among different forms of immunotherapy, redirecting T cells to hematological malignancies with bispecific antibodies (BsAbs) is an attractive strategy. BsAbs are an “off-the-shelf” product that is easily scalable in contrast to adoptive T-cell therapies. Among these, the bispecific T-cell engager blinatumomab has emerged as the most successful BsAb to date. It consists of 2 single-chain variable fragments specific for CD19 present on B-cell malignancies and CD3 expressed on almost all T cells. Blinatumomab has shown potent antitumor activity as a single agent, particularly for acute lymphoblastic leukemia, resulting in its US Food and Drug Administration approval. However, although successful in inducing remissions, these are normally short-lived, with median response durations of <1 year. Nevertheless, the success of blinatumomab has reinvigorated the BsAb field, which is bustling with preclinical and clinical studies for not only B-cell–derived lymphoblastic leukemia and lymphoma but also acute myeloid leukemia and multiple myeloma. Here, we will review the successes and challenges of T-cell–targeted BsAbs for the immunotherapy of hematological malignancies with special focus on conducted clinical studies and strategies to improve their efficacy.
Introduction
Immunotherapies that redirect T cells toward hematological malignancies for therapeutic intent have resulted in impressive clinical responses for B-cell–derived malignancies. Examples include the infusion of T cells that are genetically modified ex vivo to express CD19-specific chimeric antigen receptors (CARs) or the infusion of bispecific antibodies (BsAb) that redirect T cells to CD19.1-7 Although the majority of CD19-specific CAR T-cell therapy studies have been conducted with patient-specific products that require significant logistics and infrastructure, BsAbs, the focus of this review, are genuine “off-the-shelf” products.
More than 50 years ago, the concept of antibodies with mixed specificity was developed,8 and in the mid-1980s, the first BsAb that specifically redirected T cells to a target antigen was generated.9 Since then, the BsAb field has gradually expanded, with >2500 publications in PubMed in 2017. Numerous BsAb formats to redirect T cells to tumor antigens have been generated and are undergoing preclinical and clinical testing. Among them, bispecific T-cell engagers (BiTEs) have garnered particular interest. BiTEs consist of 2 single-chain variable fragments specific for CD3 (expressed on almost all T cells) and a tumor antigen. The CD19-specific BiTE blinatumomab has shown impressive clinical results for CD19-positive B-cell malignancies, resulting in its US Food and Drug Administration (FDA) approval in 2014.
Blinatumomab's success has fueled renewed interest in the field, both by academic institutions and pharmaceutical or biotechnology companies.10 However, preclinical and clinical studies also highlighted challenges for BsAbs. This includes biodistribution, the inhibitory tumor microenvironment, and the emergence of antigen-loss variants. Here, we will review the current status of BsAbs that redirect T cells to hematological malignancies and delineate strategies to improve their antitumor activity.
BsAb design
By bridging T cells and target cells with a BsAbs, T-cell activation is major histocompatibility complex unrestricted and no longer depends on the native T-cell receptor specificity of the activated T cell. The concept of linking T cells and target cells with a small molecule is seemingly simple. However, several factors have to be considered so that an optimal immunological synapse for T-cell activation is formed.11,12 Although proof-of-concept studies were performed by chemically crosslinking 2 monoclonal antibodies (mAbs) to generate BsAbs, the majority of BsAbs are currently generated by recombinant DNA technology.
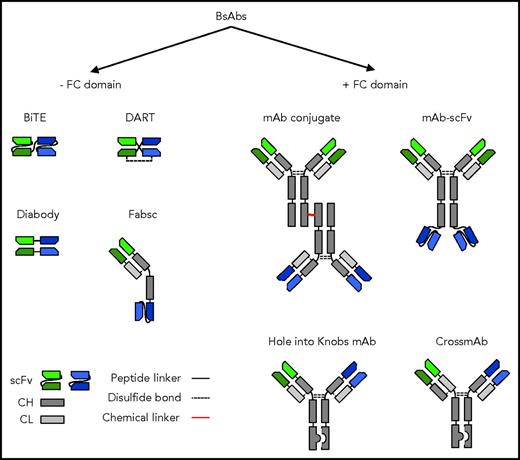
BsAbs can be divided into 2 large groups based on the presence or absence of an Fc domain. The Fc domain facilitates purification, adds stability, and increases the half-life of the molecule in vivo.13 It induces activation-dependent cell-mediated cytotoxicity (ADCC) by recruiting natural killer cells and/or macrophages.13,14 In addition, it mediates complement-dependent cytotoxicity by fixing complement once the antibody is bound to target cells.13,14 However, Fc domain-containing BsAbs might also induce ADCC of T cells once BsABs have bound to the T-cell surface, similar to CARs with Fc domains.15 This problem can be mitigated by utilizing Fc domains with mutated Fc receptor–binding sites.15 Whereas Fc-containing BsAbs are generally encoded by 2 polypeptides, BsAbs without Fc domains have been generated from a single polypeptide. Mispairing of heavy and light chains has been a significant problem when BsAbs are assembled from 2 polypeptides, and several strategies have been developed to mitigate this,13,14,16-18 including “knobs-into-hole” technology to prevent heavy-chain mispairing and cross-mAb technology to prevent light-chain mispairing.13,14,16-18 Several BsAb formats are highlighted in Figure 1. The design of BsAbs has been recently reviewed in detail elsewhere, and we refer the interested reader to the provided references, which include detailed figures depicting >100 different BsAb formats, affectionately termed the “zoo of BsAbs”14 by Brinkman and Konterman.13,14,16-18 Although it has been shown for a particular antigen that one BsAb format is superior to others,19,20 one size will most likely not fit all, because there is an intricate interplay among MAb affinity, epitope location within the targeted antigen, and antigen density and mobility on the target cell surface, all of which contribute to optimal T-cell activation.
Selected BsAb formats. BsAbs can be broadly divided into molecules that contain or do not contain an immunoglobulin G backbone with a functional Fc domain. BsABs can be created by chemical crosslinking 2 mAbs or recombinant DNA technology. Fab, fragment antigen binding; CH, heavy chain; CL, light chain; DART, dual-affinity retargeting; sc, single chain; scFv, single-chain variable fragment.
Selected BsAb formats. BsAbs can be broadly divided into molecules that contain or do not contain an immunoglobulin G backbone with a functional Fc domain. BsABs can be created by chemical crosslinking 2 mAbs or recombinant DNA technology. Fab, fragment antigen binding; CH, heavy chain; CL, light chain; DART, dual-affinity retargeting; sc, single chain; scFv, single-chain variable fragment.
Target antigen selection
The same rules for target antigen selection apply to BsAbs as for other immunotherapy approaches. Ideally, the target antigen should be (1) expressed only on malignant cells and not on normal tissue to prevent on-target/off-cancer toxicity and (2) intimately involved with the malignant phenotype to reduce the risk of antigen loss variants. Unfortunately, there are few antigens that fulfill both of these criteria. In addition, BsAb target antigens have mostly been those with cell surface expression, limiting the repertoire of antigens that can be targeted. However, mAbs have been developed that recognize a peptide/major histocompatibility complex, allowing the targeting of intracellular proteins such as Wilms tumor 1 (WT1).21 BsAbs have been developed for a broad range of hematological malignancies that are summarized in Table 1.20-34 For B-cell malignancies, CD19 and CD20 have been targeted using BsAbs.34-47 CD19 is expressed during the entire course of B-cell development, whereas CD20 is expressed once cells have matured to the naive B-cell stage. Neither is expressed on plasma cells. Targeting both antigens results in B-cell aplasia, which is considered an acceptable complication, because patients can receive intravenous immunoglobulin until B cells have recovered. Lastly, antigen-loss variants have been observed, especially with the CD19-targeted approaches that are discussed in detail in “Antigen-loss variants.”
For myeloma, B-cell maturation antigen (BCMA), Syndecan-1 (CD138), target of Wue-1 mAb, and Fc receptor-like 5 (Fcrl5 or FcHR5) have been targeted.11,48-52 BCMA has a very restricted expression and is only expressed on malignant and normal plasma cells. In contrast, CD138 is expressed not only in plasma cells but also on normal epithelial cells, raising concerns of on-target/off-cancer toxicity. The Wue-1 antibody recognizes plasma cells, but the target antigen has not been identified, which currently limits clinical development. Fcrl5 or FcHR5 is expressed in plasma cells but, in contrast to BCMA, is also expressed on naive and memory B cells.
CD33, CD123, C-type lectin-like molecule-1 (CLL-1), and WT1 have been explored as targets for myeloid malignancies. Although CD33, CD123, and CLL-1 are myeloid-lineage–specific antigens, they are expressed on either myeloid progenitor cells (CD33 and CD123) or mature neutrophils (CLL-1). WT1 is overexpressed in a broad range of malignancies, including acute myeloid leukemia (AML). It is expressed in normal kidneys and gonads; however, the infusion of WT1-specific T cells has an encouraging safety profile,53 warranting further active exploration of WT1-specific BsAbs.
In conclusion, a broad range of target antigens have been explored for BsAbs for hematological malignancies so far. Nevertheless, there is a continued need to discover new antigens, because the majority of explored antigens are not genuinely specific for the targeted malignancy.
Clinical studies
Although this section is focused on summarizing the clinical experience with blinatumomab leading to FDA approval, clinical studies with other BsAbs will also be discussed. The outcomes of pertinent studies are summarized in Table 2.
Blinatumomab
Lymphoma
The first phase 1/2 clinical study using blinatumomab treated 76 patients with DLBCL, mantle cell lymphoma, and follicular lymphoma, resulting in an overall response rate of 69% and a median response duration of 13.5 months.1,54 The antilymphoma activity of blinatumomab has been confirmed in other studies.55 Ongoing studies involve using blinatumomab in an open phase 2/3 study for patients with recurrent/refractory aggressive B-cell non-Hodgkin lymphoma (NHL; NCT02910063), or in combination with upfront chemotherapy for high-risk DLBCL (NCT03023878).
Acute lymphoblastic leukemia
Blinatumomab received accelerated FDA approval largely based on results of a multicenter single-arm study of 189 patients with relapsed, refractory, Philadelphia chromosome (Ph)–negative B-cell ALL. Of the evaluable patients, 33% achieved CR and 10% achieved CRh, with a median OS of 6.9 months.2,56 Similar results have been obtained in a phase 3 study of 405 patients (TOWER study) in which patients with recurrent or refractory Ph-negative ALL were randomized to receive blinatumomab or standard chemotherapy.3 Blinatumomab therapy resulted in a 44% CR/CRh rate with a median OS rate of 7.7 months, which was significantly higher than for patients who received standard chemotherapy (CR/CRh; 25%; median OS, 4.0%).3 Blinatumomab monotherapy is also being explored for relapsed, refractory, Ph-positive ALL. Sixteen out of 45 patients (36%) achieved a CR/CRh within the first 2 cycles, including 4 of 10 patients that had a tyrosine kinase inhibitor resistance mutation (T315I).57
Response rates and outcomes of blinatumomab are similar for pediatric ALL.58 Ongoing pediatric studies include a phase 3 study of blinatumomab vs standard chemotherapy in pediatric patients with first relapse for high-risk ALL and a phase 4 postmarketing study to determine treatment-related adverse events in pediatric and adolescent patients with relapsed/refractory ALL receiving blinatumomab (RIALTO; NCT02187354).
Several studies have attempted to identify predictors of response to blinatumomab. Thus far, high disease burden, high frequency of circulating inhibitory regulatory T cells (Tregs), and concurrent or prior history of extramedullary disease have been demonstrated to correlate with a poor response rate.59,60 In addition, expression of PD-L1 on B-cell blasts has been shown to inhibit the activity of blinatumomab (see “Immunosuppressive tumor environment”).61 Because the median OS after blinatumomab treatment is <12 months,2,3,56 it is advisable that patients proceed to HSCT once they have achieved a CR after blinatumomab treatment to prolong remission rates. Given the short median OS, concerns have been raised in regards to the high cost of blinatumomab, an issue shared with other novel anticancer therapeutics. A recent cost analysis using response rate, event-free survival, OS, number of administered blinatumomab cycles, and HSCT rates estimated from the TOWER study suggested that blinatumomab is a cost-effective alternative to standard of care in the United States.62 Clearly, additional analyses are needed to substantiate this finding.
Lastly, the anticipated FDA approval of Novartis’ CD19-CAR T-cell product for ALL will most likely change the prescribing pattern of blinatumomab in the future. However, Novartis is initially seeking approval only for pediatric ALL. The choice of autologous CD19-CAR T cells vs blinatumomab for ALL therapy will depend on the results of future studies. If CD19-CAR T-cell therapy results in long-term remissions without the need of HSCT, then it would clearly be the superior CD19-targeted immunotherapeutic. However, if patients still require HSCT after autologous CD19-CAR T-cell therapy to achieve long-term remissions, an off-the-shelf product like blinatumomab is most likely the better choice, given that it is readily available and its manufacture is less cost and infrastructure intensive.
Multiple myeloma
Several studies have reported that multiple myeloma stem cells express CD19,63 and clinical studies are in progress to evaluate whether blinatumomab has antimyeloma activity in humans. For example, a phase 1 clinical study (NCT03173430) using blinatumomab in multiple myeloma after high-dose melphalan and autologous stem cell transplant is underway to determine the feasibility, safety, and antimyeloma activity of blinatumomab after autologous HSCT.
Other CD19-targeted BsAbs
A multicenter study determining the safety and efficacy of the CD19-specific dual-affinity retargeting drug duvortuxizumab is currently recruiting adults with B-cell ALL, CLL, DLBCL, and indolent B-cell non-Hodgkin lymphoma (NCT02454270).
CD20-targeted BsAbs
FBTA05 (Lymphomun) is a heterodimeric BsAb that recognizes CD20 and CD3. In addition, it contains a functional rat-mouse chimeric Fc domain, which has the potential to induce a very robust ADCC response. FBTA05 has been used as monotherapy or followed by donor lymphocyte infusion in the treatment of CLL, high-grade non-Hodgkin lymphoma, ALL, and posttransplant lymphoproliferative disease in adult and/or pediatric patients.40,64,65 For example, 10 pediatric patients with relapsed/refractory B-cell malignancies received FBTA05 before or after allogeneic HSCT. Of these, 3 patients had stable disease, 1 had a partial response, and 5 had a (or remained in) CR. Although the reported overall median survival (1150 days) is encouraging, definitive conclusions are difficult to make given the heterogeneous patient population, small patient numbers, and variable dosing schedules.40
A chemically crosslinked CD20/CD3 BsAb (CD20Bi) has also been evaluated in early-phase clinical studies. In these studies, the CD20Bi was not directly infused but rather mixed with ex vivo–activated autologous T cells prior to their adoptive transfer. These so-called BATs have been given to a total of 15 patients with relapsed/refractory B-cell lymphoma and 12 patients with multiple myeloma before or after autologous HSCT.66-68 Three patients also received concomitant interleukin-2 (IL-2) infusions. No dose-limiting toxicity was observed. Because BATs were given as part of an HSCT regimen, it is difficult to assess whether BAT infusion had an impact on outcome.
BsAbs targeting AML
CD123- and CD33-specific BsAbs have been or are being evaluated in clinical trials for patients with AML. One clinical study with a CD123-specific duobody (JNJ-63709178; NCT02715011) was temporarily on hold due to adverse events but is now open for accrual again. Phase 1 studies evaluating the safety and efficacy of a CD123-specific dual-affinity retargeting drug (MGD006; NCT02152956) or a CD33-specific BiTE (AMG330; NCT02520427) are in progress.
Toxicities associated with administration of BsAb
Because blinatumomab is the most widely used BsAb, its toxicity profile is well established. Many of the observed toxicities are most likely not unique to blinatumomab but are expected of all CD3-activating BsAbs. Fever, thrombocytopenia, and neutropenia are the most frequent side effects associated with blinatumomab administration. Cytokine release syndrome (CRS) and neurotoxicity are less frequent but have proven dose limiting due to severity, especially in patients with high disease burden.69,70
Frequent toxicities
Throughout the blinatumomab trials, grade 1 or 2 fever was observed in up to 75% of patients,71 neutropenia in up to 37%3 and fever and neutropenia in ∼25%. In addition, liver enzyme elevation has been reported in up to 13% of patients. Electrolyte derangements such as Grade ≥3 hypokalemia and hypophosphatemia have also been observed in ≥5% of treated patients.2,3
CRS
CRS has been observed with mAb, BsAb, and CAR T-cell infusions. It is a direct result of lymphoid and/or myeloid cell activation resulting in cytokine production, including interleukin IL-6 and interferon-γ.72 Mild CRS may only manifest itself with fever, whereas severe forms resemble macrophage activation syndrome or hemophagocytic lymphohistiocytosis.70 In patients treated with blinatumomab, CRS has been described since the first pilot trial.1 CRS generally occurs during the first few days of the first cycle, but not in subsequent cycles.73 A higher incidence of CRS was observed in patients with higher disease burden and/or those who received higher doses of blinatumomab.71,73-75 CRS correlates with a better treatment response, although the treatment response is not affected by CRS severity.76
Several algorithms have been developed to grade CRS and guide interventions based on clinical findings and laboratory studies.70,72 For blinatumomab, prophylactic dexamethasone and a stepwise blinatumomab dosing regimen have been shown to decrease the incidence of CRS.71,77 In addition, tocilizumab (IL-6 receptor mAb), which is effective for CRS after CD19-CAR T-cell therapy,4,72 has successfully been used to treat CRS post blinatumomab infusion.78
Neurotoxicity
A spectrum of neurological findings has been observed after blinatumomab administration in up to 52% of patients,2,3,58,71 with less severe symptoms, including up to grade 2 tremors (17%), and more severe ones, including seizures (2%) or encephalopathy (5%). The pathophysiology of these side effects is not well understood and needs to be further investigated.2,55,56,79 In this regard, Klinger et al have proposed a model in which exposure to blinatumomab increases adhesion of T cells, resulting in their extravasation into the perivascular space within the central nervous system and endothelial activation.79 This attracts leukocytes, including monocytes, which induce neuroinflammation, resulting in neurotoxicity. For the most part, neurological side effects have been reversible upon discontinuation of the medication and start of dexamethasone. In addition, neurotoxicity has been preventively managed by instituting a stepwise blinatumomab dosing regimen and prophylactic dexamethasone.
Improving BsAbs
Preclinical and clinical studies with BsAbs have highlighted potential limitations, including (1) immunogenicity of the infused molecule, (2) development of antigen-loss variants, (3) limited T-cell activation, and (4) the immunosuppressive tumor environment.
Immunogenicity
The sequence of the single-chain variable fragments contained in blinatumomab and other BsAbs are derived from mAbs generated in mice. Thus, infusion of these BsAbs is associated with the risk of developing human anti-mouse antibodies (HAMAs). Although rarely reported, the presence of HAMAs has been observed in pediatric patients after treatment of B-cell malignancy with a CD20-targeted BsAbs.40 Although humanizing mAbs and generating mAbs from phage display libraries that are based on human sequences are effective strategies to prevent HAMA responses, these antibodies still carry the risk of inducing anti-idiotypic antibodies.
Antigen-loss variants
Antigen-loss variants have been observed in humans when a single antigen is targeted. Blinatumomab and CD19-CAR T cells are not resistant to this immune evasion mechanism. However antigen-loss variants thus far have only been observed for CD19-positive leukemia and occur with a frequency of 10% to 50%. Because CD19 signaling contributes to the malignant phenotype of ALL, complete loss of CD19 expression has only been observed when the recurring leukemia presented as AML.80 Although CD19 is not expressed on the cell surface in antigen-loss variant B-cell lineage ALL, preservation of intracellular CD19 expression has been documented, which renders the antigen inaccessible to blinatumomab or CD19-CAR T cells. Molecular studies have revealed several mechanisms, including splice variants resulting in loss of the extracellular domain of CD19,81 mutations resulting in a conformational change of the extracellular domain of CD19,82 and loss of a chaperone molecule (CD81) resulting in intracellular accumulation of CD19.83
Several strategies are being developed to counteract antigen-loss variants, including the generation of trispecific mAbs.84 Other strategies include the infusion of 2 BsAbs targeting separate antigens (eg, CD19 and CD22) expressed on malignant cells. Lastly, developing strategies to induce antigen spreading by BsAbs so that the resident immune system recognizes ALL could prevent disease recurrence.
Limited T-cell activation
Optimal T-cell activation and proliferation requires a distinct set of signals, which consist of antigen-specific CD3ζ activation (signal 1) via T-cell receptors, CARs, or BsAbs and costimulation (signal 2). In addition, upon proper costimulation, T cells induce cytokine production (signal 3), which is critical for their expansion. Although BsAbs only provide signal 1, studies suggest that BiTEs can overcome the requirement for costimulation because of their ability to trigger the formation of a particularly strong immunological synapse.85 Nevertheless, combining CD28 or 41BB activation with BsAbs has shown to improve the effector function of T cells against targets in both in vitro and in vivo models.27,38 To provide signal 3, BsAbs also have been designed to include the cytokine IL15.86
BsAbs also have the potential to activate immunosuppressive CD3-positive T cells, such as Tregs. For example, in one study the response to blinatumomab therapy correlated inversely with the frequency of Tregs in the peripheral blood of infused patients with a predictive cut off of 8.5%.59 The inhibitory function of Tregs was confirmed by in vitro studies, demonstrating contact-dependent inhibition.59
Immunosuppressive tumor environment
Many hematological malignancies actively evade immune cell responses. Malignant cells are capable of secreting inhibitory molecules, such as transforming growth factor β, expressing ligands of checkpoint inhibitors such as PD-L1, and attracting inhibitory cells such as Tregs or M2 macrophages.61,87,88 Although the provision of costimulation and cytokines that augment BsAb-mediated T-cell activation can mitigate these T-cell suppressive mechanisms, other strategies are also being developed to more directly counteract the immunosuppressive microenvironment. For example, blockade of PD-1 and/or PD-L1 has been shown to improve T-cell responsiveness to BsAbs in vitro.61,89 In addition, a pediatric nonresponder to blinatumomab was treated with the combination of blinatumomab and pembrolizumab (PD-1 mAb) and had resultant reduction in leukemic burden from 45% to 1% detectable blasts in the bone marrow.61 Blinatumomab with nivolumab (PD-1 MAb) with or without ipilimumab (CTL4A mAb) are currently being evaluated in one clinical study (NCT02879695). Other potential combinatorial therapies include depleting Tregs with metronomic doses of cyclophosphamide90 or mobilizing AML blasts out of their hypoxic, immunosuppressive stem cell niche91 prior to BsAb administration.
Alternative delivery methods for BsAbs
Because of some of the discussed limitations, investigators are exploring genetic approaches to produce BsAbs in vivo. Conceptually, this has several advantages, including (1) BsAb production at tumor sites to overcome the need of continuous infusion, and (2) codelivery of cytokines and or costimulatory molecules to overcome the immunosuppressive tumor microenvironment.
Because of their natural tropism to tumor sites, mesenchymal stromal cells (MSCs) have been explored as a “cellular platform” to deliver BsAbs in vivo. For example, MSCs producing a tetravalent tandem diabody specific for CD19 and CD3 have shown efficacy against CD19-positive lymphoma in a xenograft model.92 MSCs may themselves be immunosuppressive, and therefore, engineering of alternate immune effectors with good fitness may additionally improve potency. We and others have explored this strategy and have engineered T cells to secrete BiTEs for the adoptive immunotherapy of hematological malignancies.93-95 In addition, we have shown that the expression of costimulatory molecules on T cells enhances BiTE-mediated T-cell activation, highlighting one of the advantages of cell-based BsAb delivery.96 Lastly, the feasibility of engineering oncolytic viruses to secrete BiTEs targeting solid tumor antigens has been shown in preclinical models; however, no data are currently available for hematological malignancies.97 Obvious disadvantages of these approaches are that they rely in part on patient-specific cell products or oncolytic viruses, which require a greater infrastructure and are more cost intensive than the production of recombinant proteins.
Conclusions
BsAbs redirecting T cells to malignant cells are starting to have an impact on the clinical management of patients with hematological malignancies, which is best exemplified by the CD19-specific BiTE blinatumomab. Ongoing studies are focused to expand indications for BsAbs to AML and multiple myeloma and on improving the efficacy of BsAbs by refining their structure, combining them with other treatment modalities, or exploring alternative delivery methods. Among different strategies explored, combining BsAbs with checkpoint blockade will most likely result in improved response rates and response duration. In addition, optimizing BsAbs with Fc domains to redirect not only T cells but also simultaneously induce ADCC and/or complement-dependent cytotoxicity holds the promise of improving efficacy. Although it took >50 years for the first BsAb for hematological malignancies to get from concept to FDA approval, we hope that in the next 50 years, we will witness a much accelerated pace of discovery and translation into the clinic.
Acknowledgments
This work was supported by the National Institutes of Health, National Cancer Institute (grant P50 CA126752), a Leukemia and Lymphoma Society SCOR grant in lymphoma (7001-14), the Alex’s Lemonade Stand Foundation for Childhood Cancer, the Damon Runyon Cancer Research Foundation, and the St. Baldrick’s Foundation.
Authorship
Contribution: M.P.V., C.L.B., and S.G. wrote the manuscript.
Conflict-of-interest disclosure: M.P.V., C.L.B., and S.G. have patents and/or patent applications in the field of cancer immunotherapy. Pertinent to this review, M.P.V. and S.G. have one patent application entitled “Engager cells for Immunotherapy,” and C.L.B. and S.G. have one patent application entitled “Combination CD123 and C-type Lectin Molecule 1 Targeted T cells for Acute Myeloid Leukemia.” M.P.V. is a consultant/advisory board member for the Rally! Foundation. S.G. is a consultant/advisory board member for Merrimack.
Correspondence: Stephen Gottschalk, Department of Bone Marrow Transplant and Cellular Therapy, St Jude Children’s Research Hospital, 262 Danny Thomas Pl, MS321, Memphis, TN 38105; e-mail: stephen.gottschalk@stjude.org.
References
Author notes
M.P.V. and C.L.B. contributed equally to this study.