Key Points
Azacitidine maintenance is feasible in intensively treated older patients with newly diagnosed AML.
Azacitidine maintenance, with adjustment for poor risk cytogenetic risk at diagnosis and platelet count at randomization, improves DFS.
Abstract
The prevention of relapse is the major therapeutic challenge in older patients with acute myeloid leukemia (AML) who have obtained a complete remission (CR) on intensive chemotherapy. In this randomized phase 3 study (HOVON97) in older patients (≥60 years) with AML or myelodysplastic syndrome with refractory anemia with excess of blasts, in CR/CR with incomplete hematologic recovery (CRi) after at least 2 cycles of intensive chemotherapy, we assessed the value of azacitidine as postremission therapy with respect to disease-free survival (DFS; primary end point) and overall survival (OS; secondary end point). In total, 116 eligible patients were randomly (1:1) assigned to either observation (N = 60) or azacitidine maintenance (N = 56; 50 mg/m2, subcutaneously, days 1-5, every 4 weeks) until relapse, for a maximum of 12 cycles. Fifty-five patients received at least 1 cycle of azacitidine, 46 at least 4 cycles, and 35 at least 12 cycles. The maintenance treatment with azacitidine was feasible. DFS was significantly better for the azacitidine treatment group (logrank; P = .04), as well as after adjustment for poor-risk cytogenetic abnormalities at diagnosis and platelet count at randomization (as surrogate for CR vs CRi; Cox regression; hazard ratio, 0.62; 95% confidence interval, 0.41-0.95; P = .026). The 12-month DFS was estimated at 64% for the azacitidine group and 42% for the control group. OS did not differ between treatment groups, with and without censoring for allogeneic hematopoietic cell transplantation. Rescue treatment was used more often in the observation group (n = 32) than in the azacitidine maintenance group (n = 9). We conclude that azacitidine maintenance after CR/CRi after intensive chemotherapy is feasible and significantly improves DFS. The study is registered with The Netherlands Trial Registry (NTR1810) and EudraCT (2008-001290-15).
Introduction
About 75% of patients with acute myeloid leukemia (AML) are 60 years of age or older.1 After intensive chemotherapy, complete remission (CR) rates in the range of 40% to 55% are generally attained, resulting in median disease-free survival (DFS) of between 6 and 12 months.2-8 The prevention of relapse is the major therapeutic challenge in older patients with AML who are in CR after intensive chemotherapy. No postremission treatment to prevent relapse has been established and generally accepted in this setting, except for the use of allogeneic hematopoietic cell transplantation (allo-HCT) for a selected group of relatively fit patients.9-11 Although there is a long-standing interest in maintenance therapies such as interleukin 2,12-14 low-dose cytarabine,15 and gemtuzumab ozogamicin16 after intensive induction treatment, the clinical benefits of such maintenance therapy have remained controversial.17
Potential candidates for maintenance treatment include hypomethylating agents such as decitabine and azacitidine, which have proven efficacy and limited extra medullary toxicity in older individuals.18-20 A small randomized study comparing decitabine (20 mg/m2 for 5 days every 4-8 weeks) with conventional care (observation, low-dose cytarabine or intensive chemotherapy) was prematurely discontinued without showing a lower relapse rate for the 20 patients in the decitabine group.21 A phase 2 study exploring decitabine maintenance (20 mg/m2 for 4-5 days every 6 weeks for 8 cycles) in 134 younger patients with AML in CR1 did not show better DFS compared with historical controls (1- and 3-year DFS, 79% and 54%, respectively).22 Various other small studies involving small numbers of patients explored azacitidine maintenance, but did not yield any conclusive data on its usefulness.23,24
Here we present the final analysis of the HOVON97 study. In this phase 3 study in older patients (≥60 years) with AML or MDS-refractory anemia with excess of blasts, subjects in CR/CR with incomplete hematologic recovery (CRi) after at least 2 cycles of intensive chemotherapy were randomly assigned to receive either azacitidine as postremission therapy or no further treatment (observation). The aim was to assess the value of maintenance treatment with respect to DFS (primary end point) and overall survival (OS; secondary end point).
Methods
Study design and treatment
In this study of the Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON97), patients who had entered CR or CRi after at least 2 cycles of remission-induction chemotherapy were randomly assigned to 12 cycles of azacitidine (50 mg/m2 subcutaneously for 5 days every 4 weeks) or to observation (no further treatment). Randomizations were balanced by minimization with the factors hospital, platelet count (<100 × 109/L vs ≥100 × 109/L) at randomization, and cytogenetic risk at diagnosis (favorable/intermediate vs unfavorable). Between 30 June 2009 and 1 December 2016 a total of 118 patients were registered in the study. Two patients were considered ineligible (1 was registered twice; the second patient had no CR/CRi), so that a total of 116 eligible patients were randomized and included in the analyses. Azacitidine was provided free of charge by Celgene. The study was approved by the ethics committees of the participating institutions, and was conducted in accordance with the Declaration of Helsinki. The HOVON97 study is registered with The Netherlands Trial Registry (NTR1810) and EudraCT (2008-001290-15). The database was locked on July 12, 2018.
Eligibility
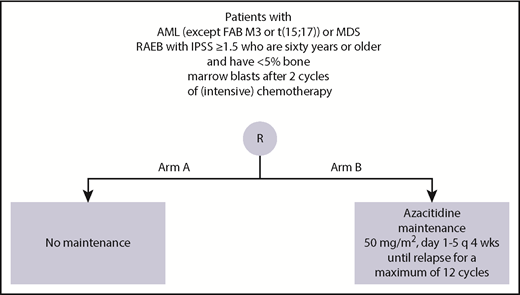
Patients with an initial cytopathologically confirmed diagnosis of AML (M0-M2 and M4-M7) and a minimum of 20% blast infiltrate in the bone marrow, who were 60 years of age or older, were eligible, provided they had a World Health Organization (WHO) performance status of 2 or less and had given their written informed consent and had less than 5% bone marrow blasts after 2 cycles of induction chemotherapy. Eligibility also included an initial subtype of the MDS (ie, refractory anemia with excess of blasts) with an International Prognostic Scoring System score of 1.5 or higher and less than 5% bone marrow blasts after 2 cycles of induction chemotherapy. Patients with extramedullary disease, AML after previous polycythemia rubra vera or primary myelofibrosis, blast crisis of CML or AML-FAB-M3, or AML with cytogenetic abnormality t15,17, and patients with a concurrent severe and/or uncontrolled medical condition or cardiac dysfunction were considered not eligible. For randomization, postremission patients were required to be in CR/CRi after at least 2 cycles of intensive chemotherapy, and to have an absolute neutrophil count greater than 0.5 × 109/L and a platelet count greater than 50 × 109/L.
Patient characteristics and classification
On the basis of karyotype at diagnosis, patients were classified into distinct prognostic categories. Patients with core binding factor abnormalities [t8,21(q22;q22), inv16(p13.1q22), or t16,16(p13.1;q22)] were classified as favorable risk. Patients without cytogenetic abnormalities or with loss of X or Y as the only abnormality were classified as normal cytogenetics. Patients with complex karyotypes [≥3 abnormalities; −5(q), −7(q), abn (3q)] were classified as unfavorable risk. The remaining patients with AML were classified as intermediate risk.
Statistical analysis, criteria of response, and evaluation of outcome
The primary objective of this study in postremission patients was to compare the value, in relation to DFS, of azacitidine therapy (intervention group) and no further therapy (observation group). DFS was measured from the date of randomization to relapse or death from any cause, whichever came first. Cox regression analysis with adjustment for the stratification factors (except center) was the primary analysis for this comparison. According to the protocol, bone marrow aspirate had to be performed after 24 weeks (observation group) or after 6 cycles (azacitidine group), and in case of suspicion of relapse (in both groups). The compliance to these bone marrow evaluations was fairly reasonable, with 7 patients (observation) vs 8 patients (azacitidine) of whom no bone marrow aspirate was performed at approximately 6 months.
Secondary objectives were to evaluate the effects of azacitidine after remission in relation to OS measured from the date of randomization, probability of relapse and death after inclusion from date of randomization (calculated as competing risks), and number and duration of hospitalizations, transfusion requirements (red cell and platelet transfusion), and adverse events. CR was defined as a cellular marrow with less than 5% blasts, no Auer rods, no evidence of extramedullary leukemia, and peripheral granulocyte and platelet counts of at least 1.0 × 109/L and 100 × 109/L, respectively. CRi was defined as CR except for residual neutropenia (<1.0 × 109/L) or thrombocytopenia (<100 × 109/L). Relapse was defined as recurrence of leukemia after CR or CRi. OS was measured from the date of registration until death from any cause. Patients known to be still alive at the date of last contact were then censored.
Based on our experience with the previous HOVON43 study, in the present study, we estimated that 40% of patients in the observation group would have a DFS of 12 months.2 We hypothesized that 60% of patients in the azacitidine maintenance group would have a DFS of 12 months. A target number of 126 patients, with 97 events required, would give a power of 80% to detect this difference with a 2-sided test at 5% significance level, an accrual period of 3 years, and an additional follow-up of 1 year.
All analyses were performed according to intention to treat, irrespective of protocol compliance. The log-rank test and Cox regression analysis were used to analyze the differences between both groups with respect to OS and DFS. These analyses were performed without and with adjustment for platelet count (<100 vs ≥100) at randomization and cytogenetic risk classification at diagnosis. All P values reported are 2-sided.
Possible heterogeneity of the treatment effects between subgroups (poor-risk cytogenetic abnormalities at diagnosis [yes vs no], platelet count [≥100 vs <100 × 109/L] at randomization, age [younger vs older than median age 69 years], cycles to CR/CRi [1 vs 2], and performance status [0 vs ≥1]) were explored. For each of the variables, a multivariate Cox regression with treatment group, variable, and treatment group × variable interaction term was performed. Only if the hazard ratio (HR) for the interaction term was statistically significant different from 1 (P < .05) were subgroup analyses performed. Otherwise, subgroup analyses were not warranted, and the estimate of the overall treatment effect also was considered the best estimate for the treatment effect within a specific subgroup.
Results
Patient cohort
The study was terminated before the accrual of the planned 126 patients. Because of declining accrual of new patients, it was estimated that the number of events, as defined in the original statistical plan, could not be reached within a reasonable time. The median follow-up time of the 116 evaluable and eligible patients still alive at the date of the last contact since the date of randomization was 41.4 months. Table 1 presents the characteristics of the patients enrolled in the observation and azacitidine maintenance groups.
Feasibility of azacitidine maintenance treatment
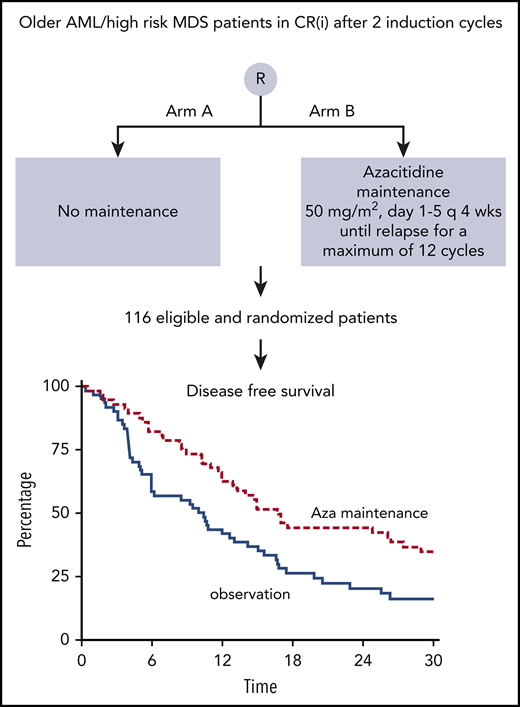
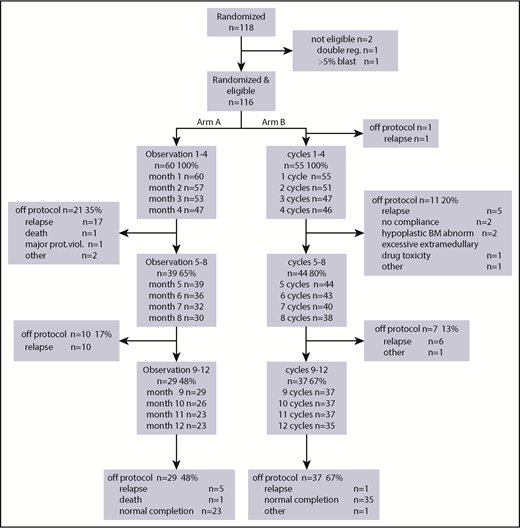
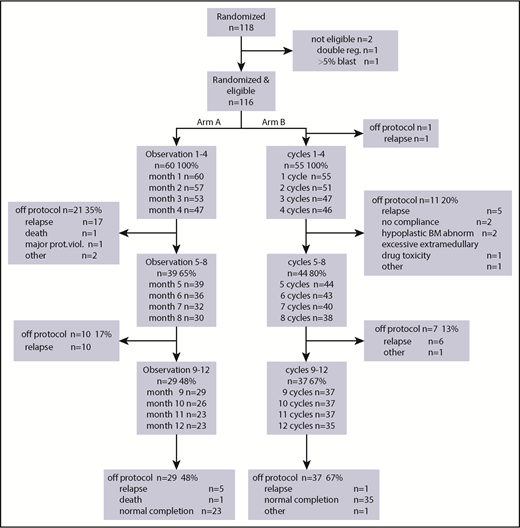
After randomization, 60 patients were assigned to the observation group and 56 patients to the azacitidine maintenance group (Figure 1). Because 1 patient had a relapse between randomization and start of azacitidine postremission treatment, 55 patients started azacitidine cycles 1 to 4. Subsequently, 44 patients started cycles 5 to 8 of azacitidine treatment, and 37 patients started cycles 9 to 12. After 4 cycles, 11 patients went off protocol (5 relapse, 2 no compliance, 2 hypoplastic bone marrow, 1 excessive extra-medullary toxicity, and 1 other reason); after 4 additional cycles, another 7 patients went off protocol (6 relapse, 1 other reason), and finally, after 12 cycles, 37 patients went off protocol (1 relapse, 1 other reason, 35 protocol completion). This is illustrated in the CONSORT flow diagram (Figure 2). Interestingly, in the azacitidine group, 35 (63%) of 56 patients completed protocol treatment, whereas in the observation group, this was feasible (ie, alive without relapse on protocol) in 23 (38%) of 60 patients.
CONSORT study diagram. Arm A, observation; Arm B, azacitidine maintenance. The main reason for failure to complete protocol was intercurrent relapse.
CONSORT study diagram. Arm A, observation; Arm B, azacitidine maintenance. The main reason for failure to complete protocol was intercurrent relapse.
The adherence to treatment according to the protocol was high. On average, 90% of the azacitidine cycles were given full dose according to schedule (mean, 90%; range, 81%-97%). The time intervals between 2 consecutive cycles was were 30 days for, on average, 86% of the cycles (mean, 86%; range, 76%-90%) and between 30 and 40 days for, on average, 10% of the cycles (median, 10%; range, 7%-14%).
Azacitidine maintenance was associated with a low transfusion dependence, a limited number of nights in the hospital, and a limited number of adverse events (AEs) and serious AEs (SAEs) (Table 2; supplemental Table 1, available on the Blood Web site). Red blood cell transfusions were not given to 92% of control patients and 86% of patients in the azacitidine group, whereas 93% and 86%, respectively, did not require any platelet transfusions. Furthermore, 92% of patients in the observation group and 86% in the azacitidine treatment group did not require clinical hospital admission. The number of AEs and SAEs were also comparable between both groups: 93% of patients in the observation group and 75% of those in the azacitidine treatment group did not experience any SAEs (ie, 4 patients in observation group and 14 patients in the azacitidine group experienced SAEs).
Treatment outcome according to postremission randomization
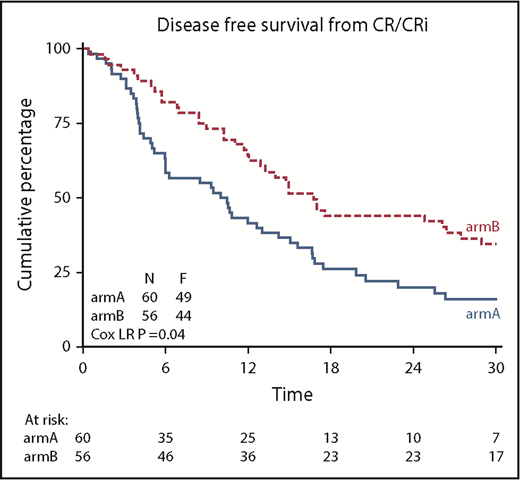
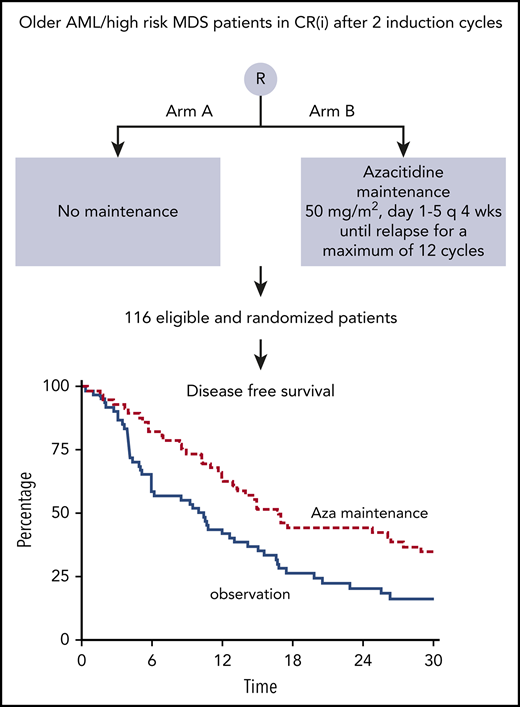
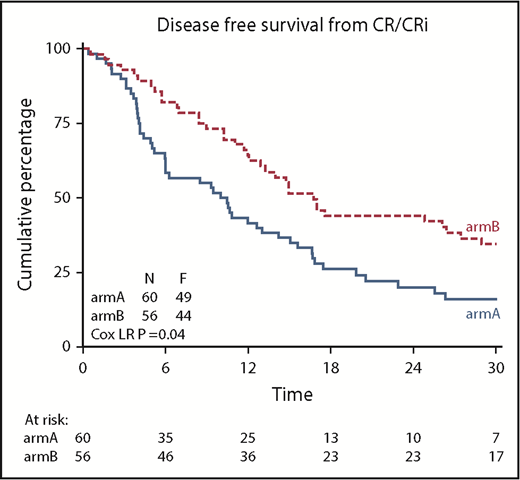
DFS was significantly improved after azacitidine maintenance treatment (64% vs 42% at 12 months; logrank; P = .04; Figure 3). DFS at 24 and 36 months was estimated at 44% and 32% for the azacitidine group and 20% and 16% for the control group, respectively. Cox regression analysis, with adjustment for poor-risk cytogenetic abnormalities at diagnosis and platelet count of at least 100 × 109/L (according to protocol), confirmed the significant improvement in DFS after azacitidine maintenance (Cox regression; HR, 0.62; 95% confidence interval, 0.41-0.95; P = .026).
Kaplan-Meier estimates for DFS. armA, observation group; armB, azacitidine maintenance group. Azacitidine maintenance treatment increased the median DFS by 5.6 months (armA, 10.3 months; armB, 15.9 months).
Kaplan-Meier estimates for DFS. armA, observation group; armB, azacitidine maintenance group. Azacitidine maintenance treatment increased the median DFS by 5.6 months (armA, 10.3 months; armB, 15.9 months).
Multivariate Cox regression analysis was performed to investigate possible heterogeneity of the treatment effects (DFS) between subgroups (poor-risk cytogenetic abnormalities at diagnosis [yes vs no], platelet count [≥100 vs <100 × 109/L], age [younger vs older than median age 69 years], response CR[i] reached after induction cycle 1 vs induction cycle 2, cycles to CR/CRi [1 vs 2], performance status [0 vs ≥ 1]). Only a statistically significant interaction between treatment group and platelet count was found, which revealed that patients with a platelet count of at least 100 × 109/L at inclusion (and not those with a platelet count <100 × 109/L) had a significant better DFS after azacitidine maintenance (supplemental Figure 2). In line with this, a significant interaction between treatment group and CR (and not CRi) was observed (supplemental Figure 3).
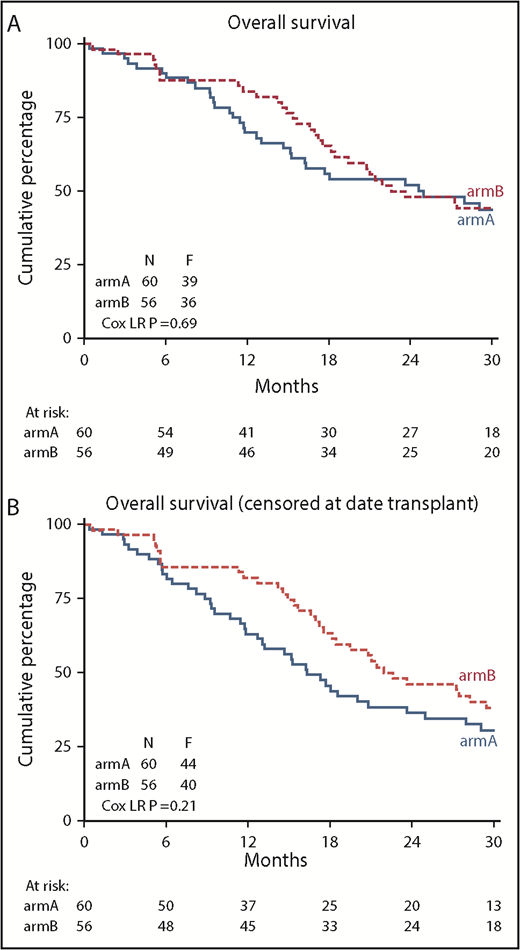
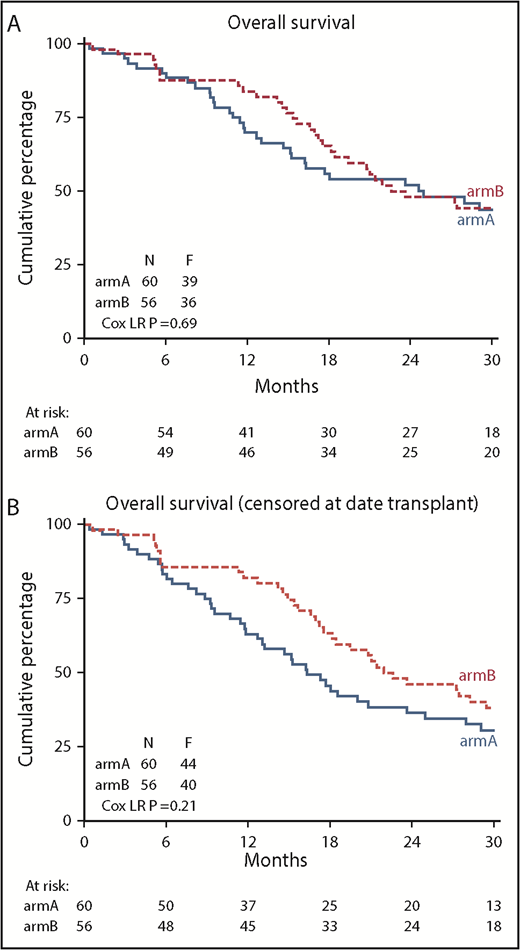
This significant improvement in DFS did not translate to a significant improvement in OS (84% vs 70% at 12 months; logrank; P = .69) (Figure 4). Cox regression analysis confirmed the lack of improvement in OS after azacitidine maintenance (Cox regression; HR, 0.91; 95% confidence interval, 0.58-1.44; P = .69). At the same time, we noted an imbalance in the use of salvage therapy after relapse between the study groups. Thirty-two patients in the control group (7 azacitidine, 19 other chemotherapy, 6 other treatment) and 9 patients in the azacitidine maintenance group (5 other chemotherapy, 4 other treatment) received rescue treatment after relapse while on protocol. In total, 87 patients relapsed until database lock (including relapses that occurred off protocol). The OS, as expected, was significantly better in those 86 relapsed patients (1 patient was lost to follow-up) who received rescue treatment (P < .001). When considering patients in each group separately, the effect of rescue treatment was observed in the relapsed patients in both the control group (P = .005) and the azacitidine maintenance group (P = .03; supplemental Figure 1). Apparently, maintenance with azacitidine did not result in resistance for rescue treatment.
Kaplan-Meier estimates of OS. (A) OS of all randomized patients (N = 116); (B) OS after censoring of 15 patients at allo-HCT (11 in arm A and 4 in arm B). arm A, observation group; arm B, azacitidine maintenance group.
Kaplan-Meier estimates of OS. (A) OS of all randomized patients (N = 116); (B) OS after censoring of 15 patients at allo-HCT (11 in arm A and 4 in arm B). arm A, observation group; arm B, azacitidine maintenance group.
Allo-HCT was used after relapse in 11 patients in the observation group and in 4 patients in the azacitidine maintenance group. After censoring patients for allo-HCT, no significant difference in OS was apparent between the groups (82% vs 63% at 12 months; logrank; HR, 0.76; 95% confidence interval, 0.49-1.17; P = .209) (Figure 4).
Discussion
The results of this study represent the first evidence from a randomized trial indicating that maintenance treatment with azacitidine significantly improves DFS for older patients with AML in CR/CRi after intensive remission-induction chemotherapy. Safety benchmarks such as protocol adherence, transfusion requirements, nights in hospital, and SAEs confirm the feasibility and efficacy of applying azacitidine maintenance treatment at a dose of 50 mg/m2 subcutaneously for 5 days every 28 days.
In general, CRs obtained after remission-induction chemotherapy in patients aged 60 years and older are short lived, and these CRs result in an OS of approximately 10% at 5 years from diagnosis. In recent years, various efforts have been undertaken to prevent relapse after CR, with limited success. Also, high-dose Ara-C consolidation schedules have failed to produce better outcomes in patients aged at least 60 years.25 Low-dose Ara-C (10 mg/m2 subcutaneously, twice daily, for 12 days) maintenance treatment has been shown to result in a modest (but significant) improvement of DFS but not OS.15 A study with rIL-2/histamine dihydrochloride showed a statistically significant benefit in DFS without a difference in OS.12 A recent study reported that maintenance therapy with norethandrolone significantly improves survival in elderly patients with AML without increasing toxicity.26 Studies evaluating gemtuzumab ozogamicin and rIL2 provided no indication for a survival advantage.12-14,16,17 Recent studies in patients aged 60 years or younger suggest benefits from maintenance therapy with the tyrosine kinase inhibitors midostaurin and sorafenib in FLT3-mutated AML.27,28 The effect of sorafenib maintenance treatment in older patients with AML has not been elucidated, as only 8 patients in the sorafenib group completed maintenance in a placebo-controlled randomized trial involving older patients with AML.29
Several previous studies have shown that azacitidine is well tolerated and has single-agent activity in AML and MDS, which was the basis for the present study on the value of maintenance treatment with azacitidine. Therefore, we considered maintenance treatment with azacitidine after intensive induction chemotherapy to be a potentially interesting option, especially for patients in CR who are not candidates for allo-HCT. Furthermore, the use of azacitidine, with its hypomethylating mechanism of action, after conventional chemotherapy, may result in antileukemic effects that are additive to the effects of chemotherapy. Increasing knowledge about the clonal hierarchy and the fact that preleukemic HSCs have been identified in remission samples, which may survive after exposure to chemotherapy, supports the concept of using azacitidine during maintenance.30,31 Indeed, our study shows that azacitidine both delays relapse and prolongs DFS in patients in CR/CRi after 2 cycles of intensive chemotherapy. These data may support the use of azacitidine in the clinical management of older patients with AML.
A question that remains is why the improvement in DFS did not translate into a significant benefit in OS. First, the trial was not powered to assess differences in OS between treatment groups. Second, the markedly greater frequency of the use of salvage treatment at first relapse in the observation group may have confounded the analysis of OS. In total, 32 patients in the control group and 9 patients in the azacitidine group received therapy after relapse while on study. It is unclear why salvage treatments were considered less often in the azacitidine group. One possible explanation is that continued treatment is less commonly considered in older patients with relapse after two different lines of treatment.
This study has several limitations, the most important being the slow accrual and early termination: 7.5 years were needed to include 118 older patients with AML in CR(i), instead of the 126 patients in the original statistical plan. Other phase 3 studies on maintenance treatment in older patients with AML also reported relatively low randomization rates.12-17 Apparently, older patients are less likely to enter treatment protocols of prolonged duration. In the current study, for instance, the burden of hospital visits needed for azacitidine injections, after extensive hospitalization for intensive chemotherapy, could have been a reason for older patients with AML to refrain from participating in the study. Therefore, the results on the safety and efficacy of maintenance with oral azacitidine, which is currently being explored in a randomized clinical trial in older patients with AML (NCT01757535), are eagerly awaited. Oral azacitidine may ensure better protocol compliance. Furthermore, the results of our study, with regard to DFS, might have been even better if the duration of azacitidine was not limited to 1 year, and azacitidine maintenance had been given until progression, in accordance with the previous studies on azacitidine treatment.19,20 Another limitation is the lack of detailed information on the molecular characterization of the AML blasts and minimal residual disease (flow and molecular). Although this is now standard practice in all HOVON AML studies, this was not common practice at the time when the HOVON97 study was planned. We were therefore unable to evaluate the effect of azacitidine maintenance within certain molecular subgroups or on reduction or elimination of minimal residual disease.
In summary, this study provides the first prospective evidence for the feasibility and effectiveness of azacitidine maintenance in newly diagnosed, intensely treated older patients with AML. It demonstrates that this therapeutic approach significantly improves DFS.
Prefinal analyses presented at the 59th annual meeting of the American Society of Hematology, Atlanta, GA, 9-12 December 2017.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the HOVON data center, especially René Hollestein, and the Data Safety Monitoring Board (N. J. G. M. Veeger, T. de Witte, and J. A. Gietema) for their enduring support for this trial. Thanks to the Dutch-Belgian Hemato-Oncology Cooperative Group for its participation in this study.
This investigator-sponsored trial was financially supported by Celgene, and they provided the azacitidine used in the trial free of charge. This study has been supported by a grant for the Dutch Cancer Foundation (KUN 2008-4291).
Authorship
Contribution: The study was designed by the Leukemia Working Group of the HOVON; the HOVON Data Center was responsible for the central data management; D.A.C. performed the analysis of the data; G.H and subsequently D.A.C., E.V., and B.L. produced the first version of the manuscript, which was circulated for comments to the other authors; and the decision to publish was made by the cooperative group.
Conflict of interest disclosure: G.H. has received research funding from Celgene; G.H. and B.L. have been involved in advisory boards for Celgene. The remaining authors declare no competing financial interests.
A complete list of the members of the Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) appears in “Appendix.”
Correspondence: Gerwin Huls, Department of Hematology, University Medical Center Groningen, University of Groningen, PO Box 30.001, 9700 RB Groningen, The Netherlands; e-mail: g.huls@umcg.nl.
Appendix: study group members
The members of the Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) who participated in this study are: D. A. Breems, Ziekenhuis Netwerk Antwerpen, Antwerp, Belgium; V. Havelange and M.-C. Vekemans, Saint-Luc University Hospital, Brussels, Belgium; G. Verhoef, University Hospital Gasthuisberg, Leuven, Belgium; B. Hodossy, Citadelle, Liège, Belgium; D. Deeren, AZ Delta, Roeselare, Belgium; C. Schuermans, GasthuisZusters Antwerpen, Wilrijk, Belgium; C. Graux, CHU UCL Namur (Godinne), Yvoir, Belgium; S. K. Klein, Meander MC, Amersfoort, The Netherlands; B. J. Biemond, Academic Medical Center, Amsterdam, The Netherlands; J. Terpstra, OLVG, Amsterdam, The Netherlands; G. J. Ossenkoppele and A. van de Loosdrecht, VU University Medical Center, Amsterdam, The Netherlands; J. W. J. van Esser, Amphia, Breda, The Netherlands; D. van Lammeren-Venema, Hagaziekenhuis, The Hague, The Netherlands; M. C. J. C. Legdeur, Medisch Spectrum Twente, Enschede, The Netherlands; E. Vellenga and G. Huls, University Medical Center Groningen, Groningen, The Netherlands; G. K. S. Jie, Atrium MC, Heerlen, The Netherlands; O. de Weerdt, St. Antonius Hospital, Nieuwegein, The Netherlands; W. van der Velden, Radboud MC, Nijmegen, The Netherlands; M. Jongen-Lavrencic and B. Löwenberg, Erasmus University Medical Center, Rotterdam, The Netherlands; J. Kuball, University Hospital Utrecht, Utrecht, The Netherlands; M. Van Marwijk Kooy, Isala, Zwolle, The Netherlands.