Background:
Outcome of Ph+ALL has changed drastically with the availability of tyrosine kinase inhibitors (TKIs). We showed that 3-month CMR is a major factor predicting overall survival (OS). However, despite the achievement of CMR, 25% of pts progressed. The aim of this analysis is to assess the prognostic factors for progression and survival, and subsequently to assess the role of allogeneic stem cell transplant (ASCT) in pts who achieved CMR within 3 months of therapy.
Methods:
We performed a retrospective chart review of 204 pts with Ph+ ALL treated, at our institution between 01/2001 and 12/2018, with the combination of hyper-CVAD (HCVAD) with a TKI; imatinib, 44 (22%); dasatinib, 88 (43%); and ponatinib, 72 (35%). Among these pts, 3-month CMR was observed in 84 (41%); 11 (25%) with imatinib; 29 (33%) pts with dasatinib; and 44 (61%) with ponatinib. Poor risk cytogenetic abnormality was defined as the presence of +der(22)t(9;22) and/or −9/9p in the absence of high hyperdiploidy (51‐65 chromosomes). Progression-free survival (PFS) was defined as the time from the start date of therapy to the date of relapse, death, or the date of last follow-up. OS was defined as from the start date of therapy to the date of death or the date of last follow-up. To identify prognostic factors for PFS and OS, we performed backward multivariate Cox regression with the variable selection with a p-value cutoff of 0.200 by univariate Cox regression analysis. Time to ASCT was handled as a time-dependent variable. We performed landmark analysis to assess the impact of ASCT on PFS and OS.
Results:
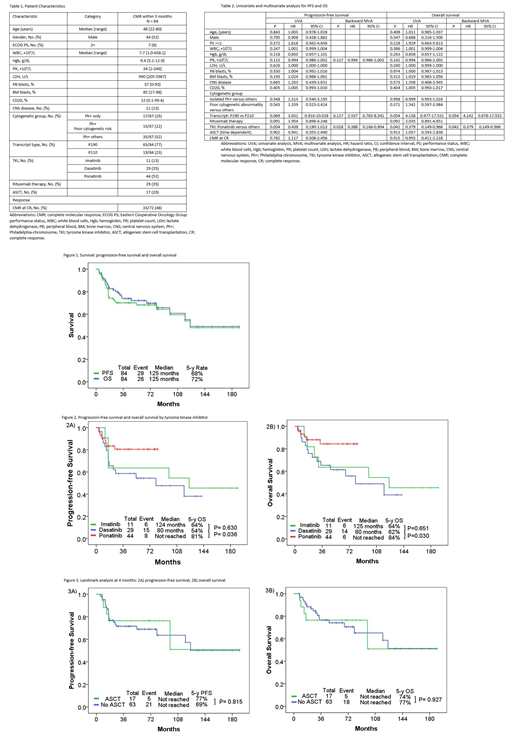
Patient characteristics are summarized in Table 1. The median age was 48 years. 47% of pts (n=44) received ponatinib; 39% (n=29), dasatinib; 13% (n=11), imatinib. 77% of pts (n=65) had P190 transcript. Only 22% (n=15/67) had poor cytogenetic abnormalities with der(22)t(9;22) or del9p/-9 in the absence of high hyperdiploidy. The median follow-up was 64 months. Overall, the median PFS and OS was 125 and 125 months, respectively (Figure 1). The 5-years PFS and OS rates were 68% and 72%. The 5-years PFS rates by TKI were 64% for HCVAD-imatinib, 54% for HCVAD-dasatinib, and 81% for HCVAD-ponatinib. The respective 5-years OS rates were 64%, 62%, and 84% (Figure 2). By multivariate analysis for PFS and OS, ponatinib therapy was the only significant independent factor predicting for progression (P=0.028; hazard ratio [HR], 0.388; 95% confidence interval [CI], 0.166-0.904) and death (P=0.042; HR=0.379; 95% CI, 0.149-0.966) (Table 2). ASCT was not prognostic factor for PFS (P=0.902; HR, 0.941; 95% CI, 0.355-2.490) and OS (P=0.913; HR, 1.057; 95% CI, 0.393-2.838) by univariate analysis. We investigated the outcome of ASCT. 17 (20%) pts (imatinib n=2, dasatinib n=8, ponatinib n=7) received ASCT in CMR within a median of 4 months. After performing a landmark analysis at 4 months, there was no difference in PFS and OS among pts who received ASCT and those who did not, regardless of the TKI used (analysis performed overall and by TKI) (Figure 3).
Conclusion: CMR predicts for the best long-term outcome. ASCT does not improve outcome once a CMR is achieved. Ponatinib is superior to other type of TKIs in inducing and maintaining CMR, and thus preventing progression.
Sasaki:Otsuka: Honoraria; Pfizer: Consultancy. Kantarjian:Agios: Honoraria, Research Funding; Immunogen: Research Funding; BMS: Research Funding; Jazz Pharma: Research Funding; Cyclacel: Research Funding; Amgen: Honoraria, Research Funding; Novartis: Research Funding; Pfizer: Honoraria, Research Funding; Astex: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; AbbVie: Honoraria, Research Funding; Daiichi-Sankyo: Research Funding; Ariad: Research Funding. Short:Amgen: Honoraria; Takeda Oncology: Consultancy, Research Funding; AstraZeneca: Consultancy. Khoury:Kiromic: Research Funding; Stemline Therapeutics: Research Funding; Angle: Research Funding. Konopleva:Astra Zeneca: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Kisoji: Consultancy, Honoraria; Ablynx: Research Funding; Agios: Research Funding; Cellectis: Research Funding; Amgen: Consultancy, Honoraria; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Forty-Seven: Consultancy, Honoraria; Ascentage: Research Funding; Eli Lilly: Research Funding; Calithera: Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding. Jain:Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics, an AbbVie company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellectis: Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Precision Biosciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. DiNardo:abbvie: Consultancy, Honoraria; celgene: Consultancy, Honoraria; jazz: Honoraria; agios: Consultancy, Honoraria; syros: Honoraria; medimmune: Honoraria; daiichi sankyo: Honoraria; notable labs: Membership on an entity's Board of Directors or advisory committees. Garcia-Manero:Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding. Kadia:Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bioline RX: Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; BMS: Research Funding. Wierda:Miragen: Research Funding; Loxo Oncology Inc.: Research Funding; Gilead Sciences: Research Funding; Acerta Pharma Inc: Research Funding; Cyclcel: Research Funding; GSK/Novartis: Research Funding; Xencor: Research Funding; Pharmacyclics LLC: Research Funding; Genentech: Research Funding; Sunesis: Research Funding; KITE pharma: Research Funding; Janssen: Research Funding; Juno Therapeutics: Research Funding; Oncternal Therapeutics Inc.: Research Funding; AbbVie: Research Funding. Daver:Hanmi Pharm Co., Ltd.: Research Funding; Servier: Research Funding; Genentech: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Jazz: Consultancy; Forty-Seven: Consultancy; Jazz: Consultancy; Daiichi Sankyo: Consultancy, Research Funding; Hanmi Pharm Co., Ltd.: Research Funding; Novartis: Consultancy, Research Funding; Immunogen: Consultancy, Research Funding; Forty-Seven: Consultancy; Servier: Research Funding; Sunesis: Consultancy, Research Funding; Astellas: Consultancy; BMS: Consultancy, Research Funding; Celgene: Consultancy; Abbvie: Consultancy, Research Funding; Agios: Consultancy; Otsuka: Consultancy; Sunesis: Consultancy, Research Funding; Glycomimetics: Research Funding; Incyte: Consultancy, Research Funding; Karyopharm: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Agios: Consultancy; Pfizer: Consultancy, Research Funding; Glycomimetics: Research Funding; Celgene: Consultancy; NOHLA: Research Funding; Karyopharm: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Otsuka: Consultancy; NOHLA: Research Funding; Abbvie: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Immunogen: Consultancy, Research Funding; Astellas: Consultancy. Thompson:Pfizer: Research Funding; Pharmacyclics: Research Funding; Genentech: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; AbbVie: Research Funding; Amgen: Consultancy, Research Funding. Cortes:Novartis: Consultancy, Honoraria, Research Funding; Sun Pharma: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Astellas Pharma: Consultancy, Honoraria, Research Funding; Forma Therapeutics: Consultancy, Honoraria, Research Funding; Biopath Holdings: Consultancy, Honoraria; Takeda: Consultancy, Research Funding; Merus: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Immunogen: Consultancy, Honoraria, Research Funding; BiolineRx: Consultancy. O'Brien:Astellas: Consultancy; Aptose Biosciences, Inc: Consultancy; Pfizer: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; Acerta: Research Funding; Alexion: Consultancy; Amgen: Consultancy; Celgene: Consultancy; Eisai: Consultancy; Gilead: Consultancy, Research Funding; GlaxoSmithKline: Consultancy; Kite: Research Funding; Janssen: Consultancy, Honoraria; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; Regeneron: Research Funding; Sunesis: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; Vaniam Group LLC: Consultancy; Verastem: Consultancy. Ravandi:Menarini Ricerche: Research Funding; Macrogenix: Consultancy, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Selvita: Research Funding; Cyclacel LTD: Research Funding; Xencor: Consultancy, Research Funding. Jabbour:Cyclacel LTD: Research Funding; Pfizer: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; BMS: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.