Background
High-resolution sequencing has improved prognostication of AML. A multistage model (MM), based on a knowledge bank (KB) of 1540 patients (pts) treated between 2004 and 2010, has been set up to improve outcome prediction and tailor therapeutic decision, including SCT (Gerstung, Nat Genet 2017). Validation of overall survival (OS) predictions by this KB has been reported (Huet, Blood 2018). Given progresses of SCT over the last decades, whether such a KB approach can be harnessed to personalize SCT decision in first remission (CR1) remains unclear. We addressed this question in younger AML pts treated intensively.
Methods
From 03/2009 to 09/2013, the multicentric ALFA-0702 study (NCT00932412) enrolled 713 pts with de novo AML aged 18-60 years old, excluding APL and CBF AML. Pts with protocol-defined non-favorable risk AML were eligible for SCT from a matched donor (Thomas, J Clin Oncol 2017). High throughput sequencing of 41 genes and ligation-dependent RT-PCR was done in 656 pts (92%). Risk stratification was assessed according to ELN 2017 (Döhner, Blood 2017). Of the 100 variables used by the MM, 3 clinical (splenomegaly, LDH and Hb levels) and 24 genetic variables (genes mutated in at most 5.3% in the KB) were unavailable. After imputation of missing data based on the KB, individualized 5y-OS predictions were done for each patient in 3 scenarios: no SCT, SCT in CR1 or SCT after first relapse, as previously described (Gerstung, Nat Genet 2017).
Results
Median age at diagnosis was 46 years. Median follow-up was 4.2 years. Overall, 302, 282 and 72 pts were never transplanted, transplanted in CR1 or after relapse, respectively (resp). ELN 2017 risk was favorable (Fav), intermediate (Int) and adverse (Adv) in 31%, 31% and 38% of the 647 evaluable pts, resp.
5y-OS was 77.7%, 58.3% and 42.2% in Fav, Int and Adv pts, resp (Fav versus [vs] Int P<.001; Int vs Adv, P=.002). When personalized KB-based OS predictions were done based on the actual timing of transplant of each patient, the accuracy of KB predictions was largely superior to that of ELN 2017 (concordance index 71.3 vs 62.5, resp).
Considering SCT in CR1 as a time-dependent variable, there was a significant interaction between ELN 2017 risk and SCT in CR1 (P<10-4), with a significant OS benefit with SCT in CR1 in Int (HR=0.54, P=0.011) and Adv (HR=0.64, P=0.023), but not Fav (HR=3.38, P=2x10-4) risk pts.
We investigated the ability of the KB approach to determine the subset of patients that may benefit from SCT in CR1 rather than at relapse (per Gerstung, Nat Genet 2017). An estimated 128 patients (ELN 2017 Fav/Int/Adv in 34.6%, 45.7% and 19.7%) would have a 10% improvement in 5y-OS if allografted in CR1. However, in these 128 pts, the effect of SCT in CR1 on OS did not differ from that in the other 528 pts (interaction test P=0.31).
We therefore sought alternative ways to inform SCT decision based on KB predictions. The KB approach predicted a 5y-OS <40% in 448 pts (ELN 2017 fav/int/adv in 16.9%, 32.5% and 50.6%, resp) in case of SCT performed at relapse. In these 448 pts, of whom 222 were transplanted in CR1, there was a significant OS benefit with SCT in CR1 (HR=0.72, P=0.02) but not in the 208 pts with a predicted OS ≥40%, of whom 60 were transplanted in CR1 (HR=2.49, P=0.01; interaction test P=0.013).
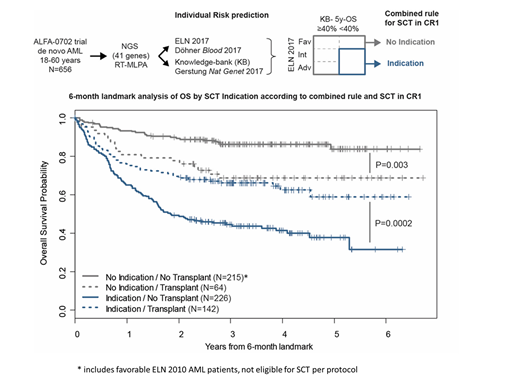
We next combined ELN 2017 risk and KB-predicted OS into a single rule to guide SCT decision. Only pts with both ELN 2017 Int/Adv risk and a KB-predicted 5y-OS <40% (N=368/647 pts, 57%) were considered to have transplant indication in CR1: 195 (53.0%) and 81 (29%) pts from the 'indication' and 'no indication' groups were transplanted in CR1, resp. In a time-dependent analysis, the 'indication' group had a significant benefit of SCT (HR=0.55, P=1.6x10-4), while the 'no indication' group had a significantly worse OS when transplanted in CR1 (HR=2.35, P=0.002; interaction test P<10-4). 211/282 SCTs in CR1 were done within 6 months from inclusion. In a 6-month landmark analysis, 5y-OS of the 142 transplanted pts from the 'indication' group was 59.0% compared to 37.9% for the 226 non-transplanted pts (P=2x10-4); 5y-OS of the 64 transplanted pts from the 'no indication' group was 68.9% compared to 83.9% for the 215 non-transplanted pts (P=0.003, interaction test P=10-5, Figure).
Conclusion
The Knowledge bank approach predicts OS of younger adults with AML more accurately than ELN 2017. ELN 2017 and KB risk predictions may be combined to optimize indications of SCT in CR1.
Berthon:JAZZPHARMACEUTICAL: Other: DISCLOSURE BOARD; PFIZER: Other: DISCLOSURE BOARD; CELGEN: Other: DISCLOSURE BOARD. Pigneux:Jazz: Honoraria; Amgen: Honoraria; Novartis: Honoraria; Abbvie: Honoraria; Roche: Honoraria; F. Hoffmann-La Roche Ltd: Honoraria; Daichi: Honoraria; Astellas: Honoraria; Pfizer: Honoraria. Recher:Sunesis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Macrogenics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees. Vey:Novartis: Consultancy, Honoraria; Janssen: Honoraria. Thomas:ABBVIE: Honoraria; INCYTE: Honoraria; DAICHI: Honoraria; PFIZER: Honoraria. Dombret:Institut de Recherches Internationales Servier (IRIS): Research Funding; CELGENE: Consultancy, Honoraria; AGIOS: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.