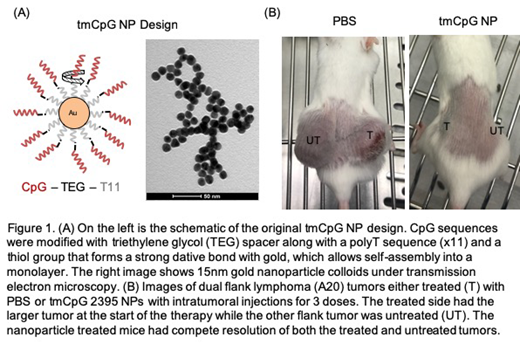
Introduction: Aggressive B cell lymphomas are clinically challenging, especially in the relapsed/refractory setting. Synthetic DNAs containing the unmethylated cytosine-phosphate-guanine (CpG) deoxynucleotides motif are potent stimulants of the innate immune system. CpGs bind to toll-like receptor 9 (TLR9) in the endosomes. Activation of TLR9 leads to G1-phase arrest of lymphoma cells and induces apoptosis via the Fas ligand pathway. Class B CpGs are linear single stranded DNA that stimulate B cells, while class C CpGs form a duplex secondary structure and act on both B cells and dendritic cells. Witzig et al. (Am J Hematol 2013) used class B CpGs in combination with radioimmunotherapy for B cell lymphomas and had an overall response rate of 93% with 63% complete responses. More recently, Frank et al. (Cancer Discovery 2018) used class C CpGs in combination with radiation to treat advanced stage indolent lymphoma and found that both treated and untreated sites had significant lymph node shrinkage. However, only 7 of 29 qualified for partial response. We have previously shown that CpG conjugated gold nanoparticles (NPs) improve macrophage stimulation. Therefore, we hypothesize that CpG-conjugated gold NPs can enhance the CpG's anti-lymphoma effects though direct and immune mediated mechanisms (Figure 1A).
Material and Methods: Using a previously verified triethylene glycol modified CpG (tmCpG) design, we synthesized class B tmCpG NPs (1826 for mice, 2006 for human) and class C tmCpG NPs (2395). Lymphoma cell lines (SUDHL4, Ramos, A20) and bone marrow derived dendritic cells (JAWSII) were used in in vitro and in vivo assays. Viability was measured by MTS and apoptosis by annexin V-PI flow cytometry. For dual tumor studies, A20 lymphoma cells were injected subcutaneously in both flanks of BALB/c mice. The side with the larger tumor was treated with either PBS, free CpG, or tmCpG NPs intratumorally on days 1, 4, and 8. Tumors were monitored for growth and, when either tumor reached 2 cm3, the mice were euthanized.
Results: Changing the CpG sequence did not alter the monolayer formation on the nanoparticles, and 1nM of tmCpG NPs are equivalent to 0.5ug/ml of CpGs. Class C tmCpG NPs (2395) tend to form aggregates during washing and collection phases but could be sonicated back into suspension. Both class B (1826 and 2006) and class C (2395) tmCpG NPs significantly reduced viability of SUDHL4, Ramos, and A20 cells, compared with free CpGs, while having no effect on the viability of JAWSII cells. tmCpG NPs induced lymphoma cell death by apoptosis as measured by annexin V-PI. In in vivo studies, both class B free CpG and class B tmCpG NP groups had reduced tumor growth and improved survival compared with PBS controls. Nine of 15 mice (60%) in the tmCpG NP group had no detectable tumor on the treated side, while only 4 of 15 mice (27%) in the free CpG group had no tumors. On the untreated side, there was no difference in tumor growth between the three groups. In another dual tumor study, the class C tmCpG NPs trended towards a reduction of tumor growth in both the treated and untreated tumors by comparison to free CpG or PBS but the sample size was too small (n=4) to achieve statistical significance. One of the 4 mice in the class C tmCpG NP group had complete resolution of both treated and untreated tumors, while all of the PBS and free CpG mice died before day 28, suggesting that there was an abscopal effect generated by using the nanoparticle construct (Figure 1B).
Conclusions: The tmCpG NP design significantly improved cytotoxicity of CpGs toward lymphoma compared with free CpGs. Class B tmCpG NPs had stronger direct cytotoxic effects at the treated tumor site compared with free CpG, while class C tmCpG NPs had immune effects at the distant untreated sites. Future studies should focus on re-engineering the platform to stabilize and optimize tmCpG NPs for improved anti lymphoma activity.
Karmali:Gilead/Kite; Juno/Celgene: Consultancy, Speakers Bureau; Takeda, BMS: Other: Research Funding to Institution; Astrazeneca: Speakers Bureau. Thaxton:Zylem: Other: Co-founder of the biotech company Zylem. Gordon:Juno/Celgene: Other: Advisory Board, Research Funding; Gilead: Other: Advisory Board; Zylem LLC: Other: co-founder; research in nanoparticles in cancer; Bayer: Other: Advisory Board.
Author notes
Asterisk with author names denotes non-ASH members.