Induction of fetal hemoglobin (HbF, α2γ2) via genome editing-mediated disruption of DNA regulatory elements that repress expression of γ-globin genes (HBG1 and HBG2) is a promising therapeutic strategy for b-hemoglobinopathies including sickle cell disease (SCD) and β-thalassemia. Optimal technical approaches and safety profiles are yet to be fully defined. We used CRISPR/Cas9 to target a DNA repressor element near the distal CCAAT box of the HBG1/HBG2 promoters. This region contains a "TGACC" motif recognized by BCL11A, a transcriptional repressor protein that regulates γ-to-β globin switch after birth. Rare germline variants at or near this motif are associated with hereditary persistence of fetal hemoglobin, a benign genetic condition that alleviates the clinical manifestations of co-inherited b-hemoglobinopathies. Previously, we showed that transduction of human CD34+ cells with lentiviral vector encoding Cas9 and guideRNA (gRNA) targeting the HBG1/HBG2 promoter caused induction of HbF in red blood cell (RBC) progeny generated in vitro (Traxler et. al, Nature Medicine v22,2016). Here we present a clinically tractable approach for disrupting the HBG1/HBG2 BCL11A binding site in human hematopoietic stem cells (HSCs).
Electroporation of Cas9:gRNA ribonucleoprotein (RNP) complex into healthy or SCD donor CD34+ cells resulted in up to 80% on-target insertion-deletion (indel) mutations and 35% HbF in erythroid progeny generated in vitro. Sixteen to 17 weeks after transplantation of gene edited CD34+ cells into immunodeficient NBSGW mice, up to 75% of donor CD34+ cells in recipient bone marrow contained on-target indels, demonstrating efficient modification of repopulating human HSCs. No differences in CD34+ cell regeneration or differentiation into erythroid, T, B, or myeloid cell lineages were observed between edited and control cells. Moreover, up to 78% of gene edited erythroid cells stained with anti-HbF antibody ("F-cells") compared to 15% in control erythroid cells, suggesting a "pan-cellular" pattern of HbF expression after editing. Strikingly, human donor-derived erythroid cells in recipient bone marrow expressed up to 40% HbF compared to 3% HbF in controls. Although the editing frequencies of HBG1 and HBG2 promoters varied between different donor CD34+ cells, an engineered variant of Cas9 containing 3 nuclear localization sequences (Wu et. al,Nature Medicine v25, 2019) edited repopulating HSCs more efficiently and consistently than conventional Cas9 with two nuclear localization signals.
Simultaneous on-target RNP-induced DSBs at both HBG1 and HBG2 can result in the deletion of the intervening 4.9-kb region, leaving a single hybrid gene with HBG2 promoter sequences fused to the downstream HBG1 gene. We detected this deletion in approximately 30% of edited cells, with no associated decline in HbF expression determined by clonal analysis of erythroid colonies. No off-target mutations were detected by targeted sequencing of the 26 top candidate sites identified by CIRCLE-seq, an in vitro genome-scale method for detecting Cas9 activity. Analysis of gene edited human donor cells purified from mouse bone marrow showed no chromosomal rearrangements by G-banding (n=20) or fluorescence in situ hybridization with a probe located distal to the HBG1/HBG2 loci (n=225).
Taken together, our studies provide novel and essential preclinical evidence supporting the safety, feasibility, and efficacy of a CRISPR-Cas9 genome editing approach to induce HbF for treating hemoglobinopathies.
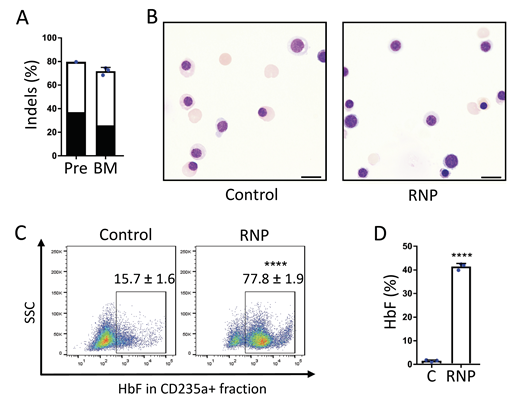
Figure. Gene editing of the HBG1/HBG2 promoters in HSCs and HbF induction of erythroid progeny in vivo. Plerixafor-mobilized CD34+ cells from an individual with SCD were edited with RNP and transplanted into NBSGW mice, which were analyzed 16-17 weeks later. A. On-target indel frequency before (Pre) and after bone marrow transplantation (BM). The black bars represent a 13-nucleotide deletion associated with human hereditary persistence of fetal hemoglobin. B. Human erythroblasts and reticulocytes derived from RNP-edited and non-edited Control CD34+ donor cells. Scale bar = 10 mm. C. HbF immunostaining control and RNP edited erythroid cells in recipient bone marrow assessed by flow cytometry. D. %HbF protein in hemolysates of control (C) and RNP edited erythroid cells assessed by ion-exchange HPLC. n= 3 biological replicates. **** P < 0.0001.
Metais:MBIO: Other: St. Jude Children's Research Hospital has an existing exclusive license and ongoing partnership with Mustang Bio for the further clinical development and commercialization of this XSCID gene therapy. Sharma:Doris Duke Foundation: Research Funding; Vertex Pharmaceuticals: Other: Study PI. Weiss:Beam Therapeutics: Consultancy; Rubius INC: Consultancy; GlaxoSmithKline: Consultancy; Cellarity INC: Consultancy; Esperian: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.