Background: Immune thrombocytopenia (ITP) is an immune mediated bleeding disorder characterized by isolated thrombocytopenia. ITP can have a variety of presentations from asymptomatic to life threatening bleeding. Although childhood ITP is most often a self-resolving illness which can be closely observed without intervention, it can be associated with significant impact on quality of life (QoL). Prospective studies of QoL in ITP patients show that there is not always a correlation with treatment or disease severity. The pathway from initial presentation to final diagnosis varies and may include encounters with emergency room, primary care or specialty providers. There have been no published studies to date showing the impact of factors prior to the diagnosis of ITP on treatment decision making and QoL.
Objective: To identify the role of physician-patient and physician-caregiver interactions on the QoL and emotional well-being of patients and their families. Ascertaining the impact of pre-diagnosis factors may provide an opportunity to improve access and quality of care provided.
Methods: The ITP Consortium of North America (ICON) "Pathways" study was a multicenter observational prospective cohort study focused on the pathways to diagnosis of ITP. The study was supported by a Foundation for Morristown Medical Center Research Fund Grant. Subjects were included if they had presumed primary ITP and were age >12 months to <18 years. Subjects were excluded if they had secondary ITP, including Evans syndrome. Treatment was determined by the physician. Subjects were consented and presented with questionnaires to be completed at the conclusion of the initial hematology visit. The hematologist also completed survey data at that time. Survey data forms included demographic form, physician form, Peds QL Family Impact Questionnaire, Kids ITP tools (KIT) Parent Impact Report and parent proxy report, and child (patient) KIT self-report. There was a parent questionnaire which included a question about worry with a scale from 0 to 10. Study data were collected and managed using REDCap electronic data capture tools hosted at Atlantic Health System. Correlation between variables were calculated using Pearson coefficient or Spearman's rho depending on the distribution of the data variables.
Results: Sixty subjects and caregivers were enrolled at 6 ICON centers; 52 were eligible for inclusion. The majority (40%) had Grade 1 bleeding. Most patients (82%) were seen in outpatient hematology clinic by the hematologist and had been referred by the emergency room (73%). The median time to consultation with a hematologist from onset of symptoms was 7 days (1-199) and the median time to diagnosis by hematologist from initial contact with a health care provider was 5 days (0-154). Most subjects had seen 2 health care providers prior to the hematologist. KIT proxy report cumulative scores were a mean of 76.03 (SD 14.72).
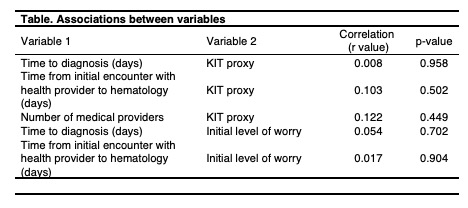
There was no significant difference between the time to diagnosis or the time from initial encounter with health care provider to hematologist and initial level of worry (p=0.70 and 0.90, respectively). There was also no significant difference between the time to diagnosis or the time from initial encounter with health care provider to hematologist and KIT proxy scores (p=0.96 and 0.50, respectively). However, there was a significant decline in level of worry (scale 0-10) prior to the hematologist visit (median 8, range 1-10) to after the visit (median 4, range 1-10). The association between number of medical providers encountered prior to diagnosis and KIT proxy scores was not significant (p=0.45) (Table).
Conclusions: In this study at 6 teaching institutions, we were unable to detect a significant difference in proxy-reported KIT scores relative to the number of health care providers seen or time from diagnosis until the first encounter with the hematologist. We were, however, able to detect a significant change in the level of caregiver worry pre- and post- visit with the pediatric hematologist, supporting a benefit of specialist care to the caregivers of children with ITP. This study was limited by its small sample size and retrospective design. ITP is considered a benign disease but is associated with a significant amount of worry and impact on QoL for patients and caregivers which warrants further investigation.
Lambert:CSL Behring: Consultancy; Amgen: Consultancy, Other; Bayer: Other: Ad boards; Novartis: Other: Ad boards, Research Funding; Shionogi: Consultancy; Kedrion: Consultancy; Sysmex: Consultancy; AstraZeneca: Research Funding; PDSA: Research Funding. Grace:Agios Pharmaceuticals, Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.