Cell surface antigen expression in AML is becoming focus of investigation as it relates to prognostic impact as well as potential therapeutic implications. The CD117 (c-KIT) receptor tyrosine kinase is expressed on the cell surface of hematopoietic stem cells and its signaling is important in cell survival, proliferation and differentiation. CD117 is expressed in a majority of AML cases. In addition, mutations in c-KIT have been identified in a subset of patients, specifically those with CBF AML (inv(16)/t(16;16) and t(8;21)). As there is increasing interest in using tyrosine kinase inhibitors with anti-KIT activity based on surface CD117 expression as treatment in AML, we sought to evaluate the association of CD117 expression with biologic and clinical features in pediatric and young adult (YA) AML.
A total of 1803 pediatric and YA patients (ages 0.01-29.2 years) treated on the Children's Oncology Group (COG) phase III trials AAML0531 (n=762) and AAML1031 (n=1041) with complete cyto/molecular and outcome data available were included in the analysis. The primary aim of AAML0531 evaluated the addition of gemtuzumab ozogamicin to a 5-course MRC-based backbone, while AAML1031 evaluated the addition of bortezomib to a similar 4-course backbone. Outcomes between the 2 primary randomized arms on each trial were similar and thus were included together for each trial. Multidimensional flow cytometry was used to determine the CD117 mean fluorescence intensity (MFI) of myeloid progenitor cells as defined by CD45 low and side scatter using a CD117-PE antibody with stable intensity detection since 2002. Patients were divided into quartiles based on CD117 expression and clinical characteristics and outcome were evaluated across the quartiles.
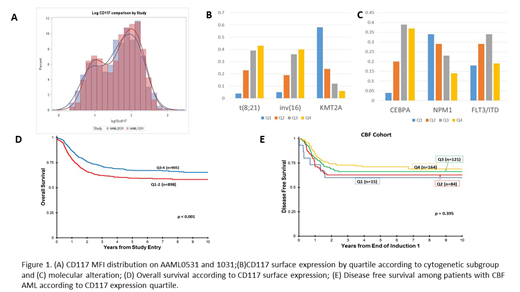
CD117 MFI distribution was similar across both trials (Fig 1A). Analysis of CD117 expression with cytogenetics demonstrated that t(8;21) was significantly associated with higher CD117 (p<0.001), with 72% of patients demonstrating expression above the median (Fig 1B). Similarly, inv(16) was associated with higher CD117 (p<0.001), with 76% of patients with CD117 MFI above the median (Fig 1B). In contrast, CD117 expression was inversely associated with KMT2A rearrangements (p<0.001), with 82% of patients with CD117 MFI below the median (Fig 1B). Evaluation of CD117 expression according to risk stratifying mutations demonstrated higher expression among patients with CEBPA mutations, with 76% of CEBPA-mutant patients with CD118 MFI above the median (Fig 1C). In contrast, CD117 expression was inversely associated with NPM1 mutations (p<0.001) as 63% of patients had expression below the median (Fig 1B). Significant differences in CD117 expression across all the quartiles was seen among FLT3/ITD patients, with the majority of patients (63%) in Q2-Q3, with 18% and 19% in Q1 and Q4 respectively (0<0.001; Fig 1C), and a similar pattern was seen among the HAR subset.
Outcome analysis demonstrated that CD117 surface expression was not associated with CR, with similar CR rates across the quartiles (p=0.726). In a univariate analysis, overall survival (OS) was associated with higher CD117 expression (p<0.001), with higher OS in Q3-Q4 (65% and 70% respectively) compared to Q1 and Q2 (61% and 58% respectively; Fig 1D). Similarly, improved disease free survival (DFS) was associated with higher CD117 expression, with higher DFS in Q3/Q4 (58% and 60% respectively) compared to 48% each in Q1 and Q2 (p<0.001). This was driven by a higher relapse risk in Q1/2 (45% each) compared to Q3/4 (39% and 36% respectively; p=0.001). Analysis in the CBF cohort demonstrated no significant differences in outcome (p=NS in OS, DFS, and relapse risk) across the quartiles (Fig 1E).
We show here that CD117 expression was higher in patients with favorable risk CBF AML and inversely associated with KMT2A rearrangements, which are generally considered to be neutral or unfavorable depending on the specific translocation partner. Higher CD117 expression was associated with improved outcomes, which may in part be explained by the association with CBF and inverse association with KMT2A. Although patients with higher CD117 MFI generally have favorable risk genomic alterations, the strategy of c-KIT inhibition warrants further investigation in AML given the potential for facilitating de-escalation of conventional therapeutics with significant toxicity in future clinical trials if found to be safe and effective.
Eidenschink Brodersen:Hematologics, Inc: Employment. Pardo:Hematologics, Inc: Employment. Dai:Hematologics, Inc: Employment. Loken:Hematologics, Inc: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.