Background: A regimen comprising of fludarabine, cytarabine, G-CSF (FLAG) has been our frontline treatment for patients with core binding factor acute myelogenous leukemia (CBF-AML) since 2007, initially in combination with gemtuzumab ozogamicin (FLAG-GO) (at 3 mg/m2 on day 1 in induction and and in 2 of the planned 6 post-remission cycles) and after withdrawal of GO from US market in combination with idarubicin at 6 mg/m2 on days 3 and 4 in induction and in one post remission cycle during cycles 3-6 (FLAG-Ida). Fusion transcripts, RUNX1-RUNX1T1 or CBFB-MYH11, were assessed at base line and monitored every 2-3 months by quantitative reverse transcriptase polymerase chain reaction (qRTPCR) during consolidation therapy.
Results: Between April 2007 and January 2018, 162 patients (Inv 16 =84, 8;21= 78) with newly diagnosed CBF-AML have been treated, median age 49 years (range, 19-78 years, 15% older than 65 years). Fifty-seven (35%) patients were treated with FLAG-GO. Amongst all patients, 95% (N=154) achieved complete remission (CR), 6 (4%) achieved CR with incomplete platelet recovery (CRp) and 2 patients died within first 4 weeks. Of the planned total of 7 cycles of therapy, median number of cycles of therapy delivered is 5 (range 1-7); prolonged cytopenias being the limiting factor in delivery of all planned cycles. With median follow up of 6.5 years, the 5 year overall survival (OS) is 71% and relapse free survival (RFS) is 75%. Patients treated with FLAG-GO and FLAG-Ida were comparable in age, cytogenetic subgroups and presence of kinase (KIT, FLT3, RAS) mutations.
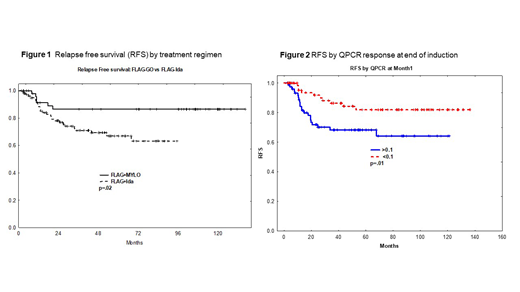
OS was not significantly different (p=.7) among treatment regimens but RFS was significantly better among patients treated with FLAG-GO (p=.02) (Fig.1). RFS at 5 years was 87% with FLAG-GO regimen while the same was 68% for FLAG-Ida regimen. Presence of KIT (p=.6) or any kinase mutation (KIT,RAS or FLT3) (p=.8) did not impact RFS. Reduction of fusion transcript ratio by 3 log at end of induction (p=.01) (Fig.2), by 4 log at end of cycle 3-4 (p=.03) and end of all cycles (p=.001), resulted in better RFS. FLAG-GO regimen but not cytogenetic subgroups was associated with better reduction in fusion transcript. Seventy-six percent of patients treated with FLAG-GO achieved reduction of fusion transcript to <0.01 by mid-consolidation while the same was 42% for patients treated with FLAG-Ida (p=0.002).
Conclusion: FLAG-GO or FLAG-Ida regimen results in high remission rates among patients with newly diagnosed patients with CBF-AML with low induction mortalities. Induction consolidation with FLAG-GO results in better RFS and quantitative reduction in fusion transcript ratio, compared to FLAG-Ida. Serial quantitative monitoring of fusion transcript identifies patients with better chances of sustained remission.
Borthakur:PTC Therapeutics: Consultancy; FTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Research Funding; Bayer Healthcare AG: Research Funding; Eli Lilly and Co.: Research Funding; Agensys: Research Funding; BMS: Research Funding; Cyclacel: Research Funding; Arvinas: Research Funding; BioTheryX: Membership on an entity's Board of Directors or advisory committees; Polaris: Research Funding; Cantargia AB: Research Funding; GSK: Research Funding; Janssen: Research Funding; AbbVie: Research Funding; Argenx: Membership on an entity's Board of Directors or advisory committees; Tetralogic Pharmaceuticals: Research Funding; Oncoceutics, Inc.: Research Funding; Oncoceutics: Research Funding; Novartis: Research Funding; Xbiotech USA: Research Funding; Eisai: Research Funding; Merck: Research Funding; BioLine Rx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; NKarta: Consultancy; Strategia Therapeutics: Research Funding. Cortes:Sun Pharma: Research Funding; Biopath Holdings: Consultancy, Honoraria; Merus: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Astellas Pharma: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Immunogen: Consultancy, Honoraria, Research Funding; Forma Therapeutics: Consultancy, Honoraria, Research Funding; BiolineRx: Consultancy; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding. Ravandi:Cyclacel LTD: Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Macrogenix: Consultancy, Research Funding; Xencor: Consultancy, Research Funding; Menarini Ricerche: Research Funding; Selvita: Research Funding. Garcia-Manero:Novartis: Research Funding; AbbVie: Research Funding; Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding. Kadia:Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Bioline RX: Research Funding; BMS: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding. Jabbour:Pfizer: Consultancy, Research Funding; Cyclacel LTD: Research Funding; Takeda: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding. Daver:BMS: Consultancy, Research Funding; Immunogen: Consultancy, Research Funding; Forty-Seven: Consultancy; Karyopharm: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Agios: Consultancy; Servier: Research Funding; Celgene: Consultancy; Astellas: Consultancy; Otsuka: Consultancy; Pfizer: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; NOHLA: Research Funding; Jazz: Consultancy; Incyte: Consultancy, Research Funding; Glycomimetics: Research Funding; Hanmi Pharm Co., Ltd.: Research Funding. Kantarjian:Daiichi-Sankyo: Research Funding; Jazz Pharma: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Ariad: Research Funding; Pfizer: Honoraria, Research Funding; Novartis: Research Funding; Takeda: Honoraria; Agios: Honoraria, Research Funding; BMS: Research Funding; Immunogen: Research Funding; Astex: Research Funding; Cyclacel: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract