Introduction: Complex aberrant karyotypes (CK, ≥3 cytogenetic aberrations, CA) are associated with an unfavorable prognosis and an increased AML transformation rate in MDS. However, even MDS with CK (CK-MDS) are heterogeneous in terms of genetic profile and prognosis. Recently, we demonstrated that a high number of CA as well as mutations in TP53 (TP53mut) are associated with increased risk in CK-MDS (Haase et al, 2019). However, as there is a strong association between CK-MDS and TP53mut, it is still a matter of debate whether the karyotype and TP53mut are prognostically independent genetic markers. Furthermore, loss of heterozygosity (LOH) of 17p13 (TP53LOH), due to loss of genetic material or to copy number neutral LOH (CN-LOH), is also associated with a poor prognosis. We here aimed to characterize TP53mut andTP53LOH in CK-MDS and to elucidate the impact of cytogenetics, TP53mut and TP53LOH on the outcome of CK-MDS.
Methods: We included 178 pts with MDS (N=138), CMML (N=5) and secondary AML after MDS (AML with myelodysplasia-related changes, N=35), all with CK. The median precentage of bone marrow (bm) blasts was 11% (range: 0-90%). The median age was 72 yrs (range: 30-95 yrs). The male:female ratio was 1.23:1. The number of CA was determined by banding analysis in all cases. The karyotype was confirmed by multicolor FISH in 134 cases. TP53LOH was verified by FISH analysis of the TP53 locus in 17p13 (146 analyses) and/or molecular karyotyping (MK, 41 analyses). In 144 cases further FISH probes in addition to TP53 were used. TP53mut was identified by NGS (54 cases) or Sanger sequencing (124 cases). Follow-up data for survival analyses were available for 127 pts with MDS and oligoblastic AML with less than 30% bm blasts.
Results: The median number of CA was 7 (range: 3-46), 98/178 pts (55%) showed a TP53mut (median VAF: 34%, range: 8-93%) and 64/178 (36%) a TP53LOH (median FISH clone size: 65%, range: 6-99%), including 9 pts with a CN-LOH in 17p13. The CN-LOH was either identified by MK (5/41 pts (12%) where MK was available showed a CN-LOH, 4/5 with TP53mut) or by NGS (4/54 pts (7%) where NGS was available showed a VAF >70% and normal TP53-FISH). In total, a TP53mut and/or a TP53LOH was identified in 116/178 pts (65%).
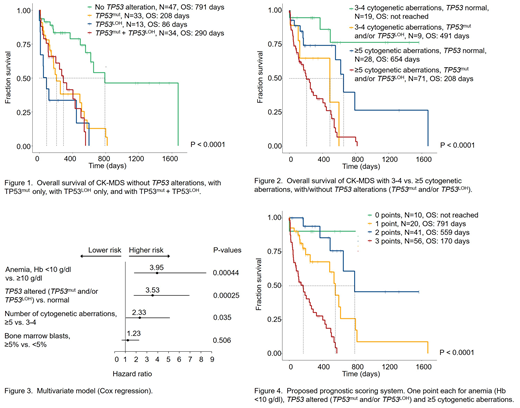
Overall survival (OS) did not significantly differ between CK-MDS with TP53mut only, TP53LOH only, and TP53mut+TP53LOH (Fig.1). Therefore, we merged TP53mut and TP53LOH to TP53altered in all further analyses. Regarding the cytogenetic characterization of pts with TP53altered, the number of CA was significantly higher in pts with TP53altered than in pts with normal TP53 (median 9 CA (range: 3-46) vs 5 CA (range: 3-24), P<0.001). Also notable was that the founder clone of pts with TP53altered included significantly more cases with del(5q) (determined by FISH, 69/92 cases, 75%) compared to pts with normal TP53 (22/52 cases, 42%, P<0.001).
The number of CA as well as the TP53 status contributed significantly to OS (Fig.2). The presence of anemia (Hb <10 g/dl) at first diagnosis of the CK had the greatest impact on OS (HR: 3.95) in the multivariate model (Cox regression), followed by an altered TP53 gene (TP53mut and/or TP53LOH; HR: 3.53) and the presence of ≥5 CA (HR: 2.33). Based on these three parameters with the greatest impact on OS, we created a simple provisional prognostic system. One scoring point each was assigned to anemia (Hb <10 g/dl), TP53altered, and ≥5 CA. Regarding OS, the resulting four risk groups separated very well (Fig.4).
Conclusions: The presence of ≥5 CA is associated with reduced OS in CK-MDS. A TP53mut as well as a TP53LOH both further segregate outcome. The impact of the clone size of TP53mut and TP53LOH on survival is currently being evaluated. Our data imply that the TP53 status (TP53mut and/or TP53LOH) and the complexity of the karyotype are independent prognostic markers. Based on the presence of anemia, the TP53 status (TP53mut and/or TP53LOH), and the number of CA, the individual risk of CK-MDS can be estimated more accurately. This will allow to better tailor treatment decisions for individual pts with CA.
Funding (FS): 2017 SGR 288-GRC
Germing:Jazz Pharmaceuticals: Honoraria; Novartis: Honoraria, Research Funding; Amgen: Honoraria; Celgene: Honoraria, Research Funding. Kaivers:Jazz Pharmaceuticals: Other: Travel Support. Kröger:Celgene: Honoraria, Research Funding; DKMS: Research Funding; JAZZ: Honoraria; Medac: Honoraria; Neovii: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Riemser: Research Funding; Sanofi-Aventis: Research Funding. Hertenstein:RS Media: Research Funding. Döhner:Daiichi: Honoraria; Jazz: Honoraria; Novartis: Honoraria; Celgene: Honoraria; Janssen: Honoraria; CTI Biopharma: Consultancy, Honoraria. Bug:Hexal: Membership on an entity's Board of Directors or advisory committees; Celgene Neovii: Other: travel grant; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees, Other: Travel grants; Sanofi: Other: travel grants; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel grants; Jazz Pharmaceuticals: Honoraria; Pfizer: Membership on an entity's Board of Directors or advisory committees. Nickelsen:Roche Pharma AG: Membership on an entity's Board of Directors or advisory committees, Other: Travel Grants; Celgene: Membership on an entity's Board of Directors or advisory committees, Other: Travel Grant; Janssen: Membership on an entity's Board of Directors or advisory committees. Platzbecker:Celgene: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Novartis: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.