Mixed phenotype acute leukemia (MPAL) is a heterogeneous disease consisting of acute leukemia expressing markers of both myeloid and lymphoid lineage. The role of hematopoietic stem cell transplantation (alloHSCT) remains uncertain due to lack of prospective trials . Several studies have retrospectively examined its role in pediatric and younger adult subgroups. Myeloablative conditioning (MAC) can result in an overall survival (OS) of 56% at 3 years, but is not suitable for older and frail patients. With expanding transplant availability and alternative donors, we sought to evaluate the outcomes of our cohort of MPAL patients, including older adults unfit for MAC, in a heterogeneous, real-world scenario compared with the outcomes of patients who were consolidated with chemotherapy only.
Methods:
Seventy four patients, aged 18 years or older, at the Princess Margaret Cancer Center between January 1, 2000 and December 31, 2018 were evaluated. All patients included in analysis met the WHO 2016 classification criteria for MPAL. Overall survival and relapse free survival rates were calculated using the Kaplan-Meier product-limit method. The log-rank test was used to compare the two groups with respect to OS and relapse free survival.
Results:
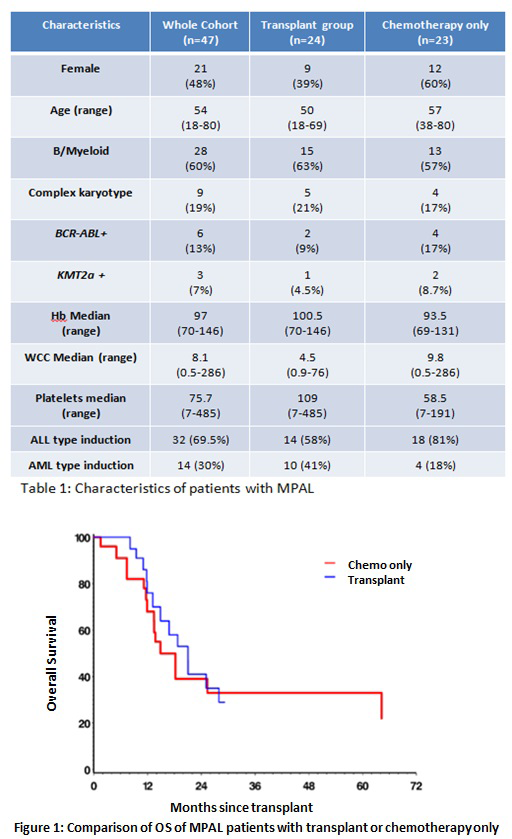
Baseline characteristics of the patient cohorts are shown in Table 1. Among the 74 MPAL patients included in this study, 59 (79%) received induction chemotherapy. Complete remission (CR) was achieved in 47 (79%) treated patients. Twenty-five of 36(80%) achieved a CR using ALL protocols (DFCI Protocol, Hyper-CVAD), while 9 of 23 (39%) achieved a CR using AML protocols (3+7, FLAG-Ida). Consolidation treatment post CR was evenly split, with 24(51%) receiving chemotherapy followed by an alloHSCT and 23 (49%) receiving chemotherapy only. In the alloHSCT group, RIC was used in 20 (81%) patients while MAC was used in four younger patients with a median age of 51 and 30 respectively. Donor types included sixteen matched unrelated donors, five sibling donors, two haploidentical donors, and one double umbilical cord. The median age of the transplant cohort was 50 years with a median OS of 21 months. In the chemotherapy only group, the median age was 57 with a median OS of 18 months. ALL-type treatment was used for consolidation in 74% of patients with 83% using the asparaginase-containing DFCI protocol.
Univariate analysis demonstrated no difference in outcomes with several parameters including age, induction chemotherapy, donor source, and conditioning intensity. The sole predictor of poor OS on univariate analysis was acute graft versus host disease (aGVHD) with a hazard ratio of 3.3 (95% CI 0.9-11). In total, 12 patients (50%) had aGVHD with a median OS of 16 months. However, when Grade 1/2 and Grade 3/4 aGVHD were compared, median OS was not reached in patients with G1/2, compared with an OS of 11 months in those patients with G3/4 aGVHD. Chronic GVHD was present in 6 patients and did not impact OS (HR 0.9892 CI 95% 0.2-2.9). Post-transplant relapses occurred solely in the RIC group and were early, occurring within a median of 124 days (range 87-188). Although, there was no relapses in the MAC group, the non-relapse mortality (NRM) was 75%, with patients dying from either aGVHD or infection. The NRM in the RIC group was 17.65%.
The 3 year OS and relapse free survival (RFS) for the entire cohort were 30% and 32%, respectively. When comparing those patients that had undergone alloSCT and those that who received chemo alone, survival in the two groups were similar with a 1 year OS of 75% vs. 68% and a 3 year OS of 29% vs 32%, respectively (Figure 1). The 3 year RFS was also similar at 29% and 34%, respectively. Subgroup analysis between the two groups was performed for patients with specific poor prognostic factors (older age, complex cytogenetics, higher WCC, conditioning regimen used), but results showed no significant survival differences.
Conclusions
Older patients with MPAL have an inferior prognosis and worse outcomes with alloHSCT compared with those of younger adults or children. Despite the increase in access to alloHSCT and an expanding pool of alternative donors, outcomes with RIC remain similar to consolidation with chemotherapy alone. There may be some evidence to suggest that the graft versus leukemia effect can be harnessed to improve outcomes in selected patients. Further research towards optimizing patient outcomes is clearly required to address the needs of older patients unfit for MAC.
Gupta:Sierra Oncology: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Honoraria, Research Funding. Mattsson:Gilead: Honoraria; Celgene: Honoraria; Therakos: Honoraria. Maze:Pfizer Inc: Consultancy; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Michelis:CSL Behring: Other: Financial Support. McNamara:Novartis Pharmaceutical Canada Inc.: Consultancy. Schimmer:Jazz Pharmaceuticals: Consultancy; Otsuka Pharmaceuticals: Consultancy; Medivir Pharmaceuticals: Research Funding; Novartis Pharmaceuticals: Consultancy. Schuh:Teva Canada Innovation: Honoraria, Membership on an entity's Board of Directors or advisory committees; Agios: Honoraria; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Teva Canada Innovation: Honoraria, Membership on an entity's Board of Directors or advisory committees. Yee:Astex: Research Funding; MedImmune: Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Hoffman La Roche: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Millennium: Research Funding; Astellas: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding. Minden:Trillium Therapetuics: Other: licensing agreement.
Author notes
Asterisk with author names denotes non-ASH members.