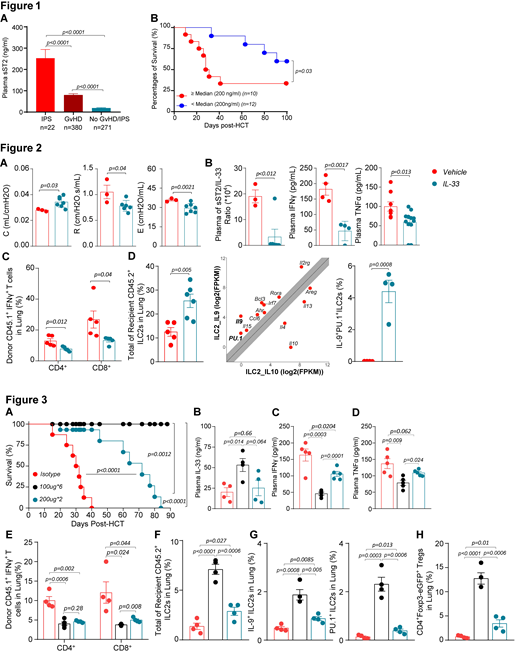
Idiopathic pneumonia syndrome (IPS) is a noninfectious acute lung injury, often fatal, following allogeneic hematopoietic cell transplantation (HCT). Similar to graft-versus-host disease (GVHD), IPS is mediated by type 1 cytopathic T cells accompanied with high levels of proinflammatory cytokines. We previously showed that elevated plasma soluble Stimulation-2 (sST2), which acts as a decoy receptor for IL-33, is a risk factor of death by GVHD (N. Engl. J. Med, 2013) or by IPS (Biol Blood Marrow Transplant, 2018). ST2 blockade of the excess of sST2 with a neutralizing antibody or small molecules released plasmatic IL-33, increasing its availability to cytoprotective T cells expressing the transmembrane molecule form of ST2, such as regulatory T cells (Tregs) reducing the type 1 proinflammatory T cell-response (Sci Transl Med, 2015; JCI Insight, 2019). The membrane-ST2 is also expressed on type 2 innate lymphoid cells (ILC2s), mostly present in lungs. Herein, we first confirmed in a cohort of 673 HCT patients that plasma sST2 measured 14 days following HCT is increased 10 fold in IPS patients (n=22) as compared to controls with no IPS/GVHD (n=271), and is 6 fold higher as compared to GVHD patients (n=380) (Figure 1A). Patients with IPS and high sST2 levels above the median of 200 ng/ml, were significantly more likely to die than patients with lower sST2 levels (Figure 1B).
In a therapeutic translational purpose, we then inquired if local administration of IL-33 via intranasal route at a dose of 500 ng/mouse daily (5 doses from day -1 to +3) will ameliorate the recipients' pulmonary function tests in a major-mismatched B6 → Balb/c HCT murine model. Allogeneic recipients that received IL-33 improved their lung compliance (C), lung resistance (R), and elastance (E) as compared to vehicle treated mice (Figure 2A). Based on our patients' data, we further explored the sST2/IL-33 ratio. Although the treatment was local, plasma IL-33 increased at day +7 post-HCT and therefore the sST2/IL-33 ratio was significantly decreased in IL-33 treated mice (Figure 2B). Parraleling this decrease, both systemic IFNγ and TNFα at day +7 post-HCT were significantly lower in mice treated with IL-33 compared to vehicle treated mice (Figure 2B). Findings in the plasma were also correlated with a local decrease of IFNγ secretion in the bronchoalveolar lavage of IL-33 treated mice (not shown). The frequencies and numbers of donor CD45.1+ IFNg+CD4+ and IFNg+CD8+ donor T cells in the lungs of IL-33 treated mice were also significantly decreased as compared to vehicle treated mice (Figure 2C). We next sought to determine if IL-33 had an impact on recipient ILC2s (CD45.2+Lin-CD90.2+GATA3+ST2+). As shown in Figure 2D, recipient mice treated with IL-33 had significant higher frequencies of lung ILC2s at day +7 post-HCT compared to mice treated with vehicle. RNA-seq analysis of sorted ILC2s from the lungs of naïve GATA3 reporter mice treated with IL-33 showed increased Il9 and PU.1 transcripts in lung ILC2s, validated at the protein level in allogeneic mice treated with IL-33 as compared to allogeneic vehicle treated mice in which ILC2s were undetectebale (Figure 2D).
As antibody (Ab) injection is more clinically relevant than local cytokine instillation, and since we have shown that anti-ST2 Ab results in IL-33 increase, we tested this in a minor-mismatched B6 → C3H.SW HCT murine model, and respectively treated mice with anti-ST2 Ab 100μg/dose every other day (6 doses total) or anti-ST2 Ab 200μg/dose for 2 doses at days -1 and +1 or isotype 100μg/dose for 6 doses. Prophylactic administration of anti-ST2 Ab with 6 doses and 2 doses significantly decreases mortality as compared to isotype with six doses allowing a better survival than the peritransplant administration (Figure 3A). Plasma IL-33 increased in both anti-ST2 treated groups vs. isotype (Figure 3B). Consistently, both plasma IFNγ and TNFα were significantly decreased in anti-ST2 Ab treated groups (Figures 3C, 3D). Percentages of cytopathic lung donor CD4+IFNγ+ and CD8+IFNγ+ T cells were decreased (Figure 3E) while cytoprotective lung recipient total, IL-9+, and PU.1+ ILC2s were increased in anti-ST2 Ab treated groups vs. isotype (Figures 3F, 3G). Tregs in both anti-ST2 Ab treated groups were concomitantly increased (Figure 3H).
We concluded that not only is sST2 a prognostic biomarker for IPS but it is also a promising therapeutic target that may prevent IPS via IL-33 induced IL-9 secreting ILC2s.
Paczesny:Viracor Eurofins Clinical Diagnostic: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.