TO THE EDITOR:
Tyrosine kinase inhibitors (TKIs) are used post-allogeneic hematopoietic cell transplantation (HCT) to reduce relapse rates in Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL).1,2 However, because results from retrospective studies are conflicting2-9 and there has been no definitive randomized trial,10 the role and duration of maintenance therapy with TKIs post-transplant remain uncertain. Herein, we present updated outcomes of patients with Ph+ ALL who received HCT followed by TKI maintenance at our institution11 and evaluate the impact of TKIs (including newer generations) on survival outcomes in light of available measurable residual disease (MRD), as assessed by quantitative polymerase chain reaction for BCR-ABL1 transcripts at the time of HCT. Furthermore, we attempt to identify the optimal duration of TKI therapy after transplantation.
A total of 171 patients with Ph+ ALL consecutively underwent first HCT at MD Anderson Cancer Center from January 2001 through December 2018. Six patients who were not in morphological complete remission (CR) at the time of HCT were excluded from the analysis. Complete molecular response (CMR) was defined as the absence of a detectable BCR-ABL1 transcript with a sensitivity of ≤0.01%.12 Major molecular response (MMR) was defined as a BCR-ABL1:ABL1 ratio ≤0.1% on the International Scale for p210 BCR-ABL1 or a 3-log reduction in transcripts of p190 BCR-ABL1, but not meeting criteria for CMR.12 Relapse was defined by recurrence of ≥5% blasts in bone marrow or by the presence of extramedullary disease.
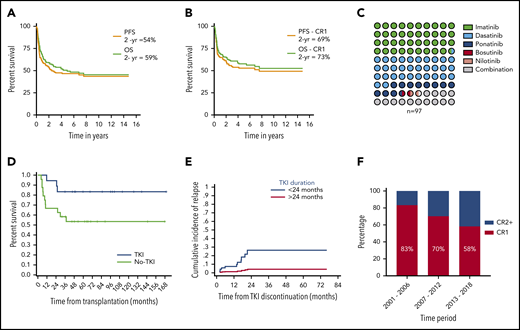
Patient demographics, baseline disease, and treatment characteristics of 165 patients are listed in Table 1. The median follow-up of the cohort from the time of HCT was 67 months (range, 7-180). At the time of the last follow-up, 88 (53%) patients had progressed. The 2- and 5-year progression-free survival (PFS) rates for the whole cohort were 54% and 46%, respectively (Figure 1A). A total of 84 (51%) patients had died at the time of the last follow-up. The 2- and 5-year overall survival (OS) rates for the cohort were 59% and 49%, respectively (Figure 1A). For patients undergoing transplantation while in CR1, PFS and OS rates were 69% and 73%, respectively, at 2 years, and 53% and 57%, respectively, at 5 years (Figure 1B). Univariate analysis showed that age >40 years (hazard ratio [HR], 1.8; 95% confidence interval [CI], 1.2-2.8; P = .006); Karnofsky performance score ≤80 (HR, 1.9; 95% CI, 1.2-3.1; P = .007), HCT-CI score 0 vs ≥1 (HR, 2.3; 95% CI, 1.2 −4.4; P = .009), and disease status at HCT advanced remission vs CR1 (HR, 1.7; 95% CI, 1.1-2.6; P = .014) were significantly associated with worse PFS. Multivariate analysis showed that age (HR, 2.56; 95% CI, 1.3-4.9; P = .004), disease status (HR, 1.927; 95% CI, 1.9-6.2; P = .0001), and Karnofsky score (HR, 1.3; 95% CI, 1.3-4.4; P = .005) retained significance. In a competing risk-regression model, the depth of MRD, as assessed by the BCR-ABL1:ABL1 transcript ratio, was a significant predictor of cumulative incidence of relapse (CMR vs others; P = .03; HR, 2.5; 95% CI, 1.09-5.71) but not for PFS (CMR vs others, P = .15; HR, 1.63; 95% CI, 0.82-3.2) in our cohort. On multivariate analysis, disease status at transplant (CR≥2 vs CR1; P = .002; HR, 3.36; 95% CI, 1.54-7.32), depth of MRD (others vs CMR, P = .03; HR, 2.5; 95% CI, 1.09-5.71) and cytogenetic status (Ph+ alone vs Ph+ other cytogenetic abnormality; P = .006; HR, 0.3; 95% CI, 0.133-0.7) were significant predictors of cumulative incidence of relapse (supplemental Table 1, available on the Blood Web site).
Study outcomes and practice patterns. (A) PFS and OS for the whole cohort of patients with Ph+ ALL. (B) PFS and OS for patients with Ph+ ALL who underwent allo-HCT while in CR1. (C) Dot matrix chart showing percentage of patients receiving different types of TKI. (D) Landmark analysis of patients who were in CMR status before and at 3 months after allo-HCT, with a significantly higher PFS in patients who received prophylactic TKI therapy compared with those who did not. (E) Graph showing increased incidence of relapse in patients who discontinued TKI therapy before 24 months vs those who continued for >24 months. (F) Decreasing percentage of patients who underwent transplantation while in CR1 over time.
Study outcomes and practice patterns. (A) PFS and OS for the whole cohort of patients with Ph+ ALL. (B) PFS and OS for patients with Ph+ ALL who underwent allo-HCT while in CR1. (C) Dot matrix chart showing percentage of patients receiving different types of TKI. (D) Landmark analysis of patients who were in CMR status before and at 3 months after allo-HCT, with a significantly higher PFS in patients who received prophylactic TKI therapy compared with those who did not. (E) Graph showing increased incidence of relapse in patients who discontinued TKI therapy before 24 months vs those who continued for >24 months. (F) Decreasing percentage of patients who underwent transplantation while in CR1 over time.
A total of 97 (59%) patients received post-transplant TKI maintenance therapy as prophylaxis (n = 71) or, at the first MRD positivity post-HCT (MRD-triggered; n = 26): imatinib (n = 38), dasatinib (n = 31), ponatinib (n = 11), bosutinib (n = 1), nilotinib (n = 1), or a combination of any 2 of those (n = 15; Figure 1C). The median time to TKI initiation after HCT was 2.4 months (range, 19 days to 35 months). The median time to TKI commencement in the prophylactic and MRD-triggered groups was 2.3 months (range, 18 days to 11 months) and 3.2 months (range, 38 days to 30 months), respectively. In an attempt to assess the impact of TKI maintenance and, within the limitations of a retrospective analysis, we performed a landmark analysis at 3 months in patients who were in CMR status before HCT and remained alive and in CMR at 3 months post-HCT (n = 42). Among these 42 patients, 18 had begun receiving prophylactic TKIs within the 3 months after transplantation (group A). The remaining 24 patients either did not receive TKI therapy (n = 16) or received therapy (either MRD-triggered or prophylactic; n = 8) at 3 months after transplantation (group B). The 2-year PFS for patients in group A was 94.5% (95% CI, 74.3%-99.0%; median PFS, 144 months) compared with 75% in group B (95% CI, 46.8%-82.0%; median PFS, 96 months; P = .041; Figure 1D).
In an effort to compare the efficacy of newer generation TKIs vs imatinib, we analyzed the relapse rates in the prophylactic and MRD-triggered groups separately. Patient whoalysis. In the TKI prophylactic group, 28 patients received imatinib, and 33 patients received newer generation TKIs. The relapse rate was similar, with 3 patients experiencing relapse in each arm. However, in the MRD-triggered group, 6 (75%) of 8 patients who received imatinib had a relapse compared with 6 (45%) of 11 patients who received newer generation TKI. Several studies have shown that the BCR-ABL1 kinase mutation is the major cause of relapse in Ph+ ALL.13,14 Newer generation TKIs overcome some of these mutations and may lead to lower relapse rates in patients with more resistant disease, as we observed in our MRD-triggered group. However, lack of mutational status and the number of patients preclude definitive conclusions from this study.
The optimal duration for continuing maintenance of TKI post-transplantation is unclear. At our center, our practice has been to continue TKI maintenance for a minimum of 2 years up to 5 years; then, treatment can be stopped at the individual physician’s discretion or if a patient develops adverse effects. To evaluate the impact of the duration of TKI and limit biases, we included patients who were alive in CMR at 3 months post-allo-HCT and continuing to take TKIs (n = 84). The median duration of TKI maintenance was 13 months (range, 0.23-74), with the median duration between stopping the TKI and last follow-up at 20 months (range, 7 days to 161 months). Of these 84, 29 continued receiving TKI for more than 24 months. On a competing risk-regression model, patients who continued TKI maintenance for more than 24 months had a significantly low risk of relapse (HR, 0.12; P = .045) compared with patients who stopped before 24 months (Figure 1E). Among the patients who received TKI therapy for more than 24 months, there was only 1 relapse.
The median and range of duration of administration for each TKI is available in supplemental Table 3. The most common reason for stopping TKI treatment was completion of the planned therapy in the absence of disease (n = 27), monitored at the molecular level by polymerase chain reaction. At the time of last follow-up, another 16 patients were continuing maintenance therapy; 21 patients stopped the therapy because of disease recurrence (supplemental Table 4). The major adverse events for which TKIs had to be stopped were decreased blood counts (n = 15); fluid retention, including edema and pleural or pericardial effusion (n = 5); recurrent infections (n = 2); gastrointestinal symptoms, including persistent nausea and vomiting (n = 7); rash (n = 2); myalgias, asthenia, and poor quality of life (n = 3); liver toxicity (n = 3); and pulmonary hypertension/right heart failure (n = 2). Another 8 patients ceased therapy because of insurance issues, noncompliance, or loss to follow-up.
Several retrospective and prospective studies2-9 have been conducted to address the question of TKI maintenance post-allo-HCT. In the only randomized trial from the German multicenter study group for adult ALL,10 55 patients with Ph+ ALL who underwent HCT were randomly assigned to receive imatinib as prophylaxis or based on MRD positivity. Although prophylactic imatinib prevented molecular recurrence, event-free survival and OS did not differ significantly between the 2 treatment arms: 5-year OS was 80% in the prophylactic group vs 75% in the MRD-triggered group. In the European Blood and Marrow Transplant registry, the Acute Leukemia Working group8 analyzed the outcomes of 473 patients with Ph+ ALL who underwent transplantation while in CR1 and received post-HCT TKI maintenance (n = 157). The post-HCT use of a TKI was associated with improved OS (HR, 0.44; P = .002), disease-free survival (HR, 0.42; P = .004), and reduced rate of relapse (HR; 0.4, P = .01).
In summary, our data demonstrate that TKI maintenance results in a lower rate of hematological relapse and improved PFS. Furthermore, the optimal duration of maintenance should be at least 2 years post-HCT. Additional studies including BCR-ABL mutation status are needed to assess the impact of newer generation TKIs.
Original data are available by e-mail request from the corresponding author.
The online version of this article contains a data supplement.
Authorship
Contribution: N.S. collected and analyzed the data and wrote the manuscript; D.M. performed statistical analyses and critically analyzed the manuscript; C.L., R.D., and G.R. collected the data; U.R.P., Q.B., C.M.H., Y.N., A.M.A., M.H.Q., S.C., E.S., I.K., H.K., E.J., F.R., and R.E.C. critically discussed the manuscript; and P.K. designed and supervised the study, analyzed the data, and revised the manuscript.
Conflict-of-interest disclosure: P.K. has received research support from Amgen and Ziopharm; has served on advisory boards of Pfizer, Kite, and Novartis; and has received consulting fees from Jazz. F.R. has received research funding from BMS, Amgen, Xencor, Abbvie, and Orsenix; has served on advisory boards of BMS, Amgen, Astellas, AstraZeneca, Celgene, Orsenix, and Agios; and has received honoraria from BMS, Celgene, Astellas, Abbvie, AstraZeneca, Novartis, Orsenix, and Agios. The remaining authors declare no competing financial interests.
Correspondence: Partow Kebriaei, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: pkebriae@mdanderson.org.