Background: Axi-cel, an autologous anti-CD19 chimeric antigen receptor (CAR) T cell therapy, is approved for treatment of adult pts with R/R LBCL after ≥ 2 lines of systemic therapy. In the pivotal ZUMA-1 study, axi-cel demonstrated durable responses and a largely manageable safety profile (Locke FL, et al. Lancet Oncol. 2019). ZUMA-9 (NCT03153462), a multicenter, open-label study, provided pts with R/R LBCL with expanded access to axi-cel until commercial availability (Cohort 1 [C1]) and later, if commercially manufactured product did not meet commercial release specification(s) (Cohort 2 [C2]). Safety and efficacy of axi-cel (C1 and C2) and translational analyses (C2) are presented.
Methods: Eligible adults had histologically confirmed R/R LBCL, ECOG ≤ 1, and received prior CD20-targeting and an anthracycline-containing regimen. C2 pts must have had commercial OOS product. Pts underwent leukaphersis and conditioning chemotherapy (cyclophosphamide 500 mg/m2/day and fludarabine 30 mg/m2/day) for 3 days followed by a single axi-cel infusion (target dose, 2 × 106 CAR T cells/kg). C1 and C2 pts with high disease burden could receive bridging therapy before conditioning at investigator's discretion. Endpoints included frequency of adverse events (AEs), objective response rate (ORR) per standard-of-care imaging assessment, overall survival (OS) for C1 and C2, and blood CAR T cells levels and serum cytokines for C2 only. Outcomes were contextualized with the primary analysis of ZUMA-1 C1+2 (n = 101; ≥ 6 mo of follow-up; Neelapu SS, et al. NEJM. 2017).
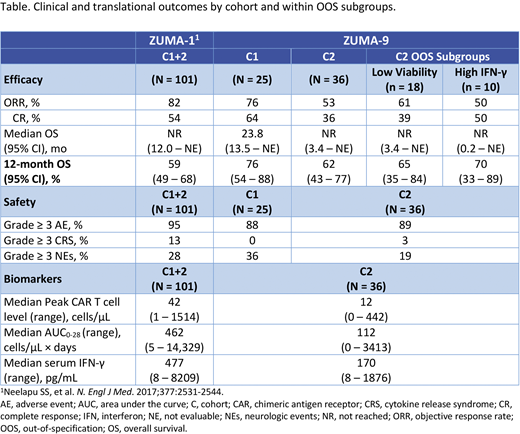
Results: As of 11/29/2019 (C1) and 3/15/2020 (C2), 25 C1 pts and 36 C2 pts received axi-cel with a median follow-up of 27.1 mo (range, 23.6 - 29.6) and 13.2 mo (range, 0.4 - 25.7), respectively. In C1, median age was 56 y (range, 28 - 76), 60% were male, 80% had DLBCL, 48%/0% had ECOG1/≥2, 44% had IPI ≥3, and 64% had ≥ 3 prior lines of therapy. In C2, median age was 61 y (range, 24 - 81), 75% were male, 78% had DLBCL, 58%/17% had ECOG 1/≥2, 56% had IPI ≥3, and 69% had ≥ 3 prior lines of therapy. In C2, 50% of pts had OOS product with low viability, 28% had high IFN-γ, 14% had low IFN-γ, 14% received a low dose, and 6% had high transduction ratio. The ORR was 76% (64% complete response [CR]) for C1, 53% (36% CR) for C2, and 82% (54% CR) for ZUMA-1 C1+2. Median OS was 23.8 mo (95% CI, 13.5 - NE) for C1 and not reached (95% CI, 3.4 - NE) for C2, respectively. ORR and OS were consistent in C2 OOS subgroups (Table).
Grade ≥ 3 AEs were reported in 88% and 89% of C1 and C2 pts, respectively. Grade ≥ 3 CRS (Lee et al, Blood. 2014) was not observed in C1 but was reported in 3% of C2 pts (13% in ZUMA-1 C1+2). Grade ≥ 3 neurologic events (NEs) occurred in 36% and 19% of pts in C1 and C2, respectively, and 28% of pts in ZUMA-1 C1+2. No Grade 5 CRS or NEs occurred in C1 or C2. All CRS and NEs resolved in C1, and most CRS (29/30) and NEs (19/24) resolved in C2 as of the data cutoff. Of 3 Grade 5 AEs in C1, 2 were unrelated to axi-cel (clostridial sepsis [on Day (D) 6] and respiratory failure [on D212]) and 1 was related to conditioning (myelodysplastic syndrome [on D563]). Of 3 Grade 5 AEs in C2, 2 were unrelated to axi-cel (multiple organ dysfunction syndrome [on D6] and cardiac arrest [on D482]) and 1 was related to axi-cel (systemic mycosis [on D30]).
Median peak CAR T cell levels and median CAR T cell expansion (area under the curve in the first 28 days) were lower in ZUMA-9 C2 (n = 32/36; 12 cells/µL [range, 0 - 442] and 112 cells/µL × days [range, 0 - 3413]) vs ZUMA-1 C1+2 (n = 96/101; 42 cells/µL [range, 1 - 1514] and 462 cells/µL × days [range, 5 - 14,329]). Serum IFN-γ levels peaked within 8 days after axi-cel infusion (median, 170 pg/mL [range, 8 - 1876]) and were lower vs ZUMA-1 C1+2 (median, 477 pg/mL [range, 8 - 8209]). Axi-cel in ZUMA-9 C2 contained fewer, less differentiated CCR7+ naïve and central memory T cells, and a greater proportion of more differentiated CCR7- effector memory and effector T cells vs ZUMA-1 C1+2. No cases of replication-competent retroviruses were reported in C1 or C2.
Conclusion: Axi-cel treatment demonstrated a manageable safety profile and meaningful clinical benefit in this expanded access and OOS product study. While CAR T cell therapy showed clinical benefit in C2 (OOS product), the lower CR rate was corroborated by lower CAR T cell expansion and a more differentiated product vs ZUMA-1 C1+2, warranting further investigation. ZUMA-9 C2 is enrolling pts and evaluation is ongoing.
Jacobson:Precision Biosciences: Consultancy, Honoraria, Other: travel support; Celgene/BMS: Consultancy, Honoraria, Other: travel support; Novartis: Consultancy, Honoraria, Other: travel support; AXIS: Speakers Bureau; Nkarta: Consultancy, Honoraria, Other: travel support; Clinical Care Options: Speakers Bureau; Pfizer: Research Funding; Lonza: Consultancy, Honoraria, Other: travel support; Kite, a Gilead Company: Consultancy, Honoraria, Other: travel support. Locke:Calibr: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Wugen: Consultancy; GammaDelta Therapeutics: Consultancy; Celgene/Bristol-Myers Squibb: Consultancy; Novartis: Consultancy; Cellular Biomedicine Group: Other: Consultancy with grant options; Allogene: Consultancy. Miklos:Adaptive Biotech: Consultancy, Other: Travel support, Research Funding; Novartis: Consultancy, Other: Travel support, Research Funding; Pharmacyclics: Consultancy, Other: Travel support, Patents & Royalties, Research Funding; Juno-Celgene-Bristol-Myers Squibb: Consultancy, Other: Travel support, Research Funding; Allogene Therapeutics Inc.: Research Funding; Miltenyi Biotec: Research Funding; Janssen: Consultancy, Other: Travel support; Kite-Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding. Vose:Incyte: Research Funding; Novartis: Research Funding; Bristol-Myers Squibb: Research Funding; Karyopharm Therapeutics: Consultancy, Honoraria; Allogene: Honoraria; Loxo: Consultancy, Honoraria, Research Funding; Seattle Genetics: Research Funding; Miltenyi Biotec: Honoraria; Janssen: Honoraria; Epizyme: Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; Kite, a Gilead Company: Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Verastem: Consultancy, Honoraria; Wugen: Honoraria; Celgene: Honoraria; Roche/Genetech: Consultancy, Honoraria, Other. Lin:Sorrento: Consultancy, Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding; Vineti: Consultancy; Gamida Cells: Consultancy; Takeda: Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Legend BioTech: Consultancy; Juno: Consultancy; Bluebird Bio: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Novartis: Consultancy; Janssen: Consultancy, Research Funding. Budde:Roche: Consultancy; Merck: Research Funding; Amgen: Research Funding; Kite, a Gilead Company: Consultancy; Gilead Sciences: Consultancy; Mustang Therapeutics: Research Funding; AstraZeneca: Research Funding. Maloney:Celgene: Consultancy, Honoraria, Research Funding; A2 Biotherapeutics: Consultancy, Current equity holder in publicly-traded company, Honoraria; Gilead Sciences: Consultancy, Honoraria; Juno Therapeutics: Consultancy, Honoraria, Patents & Royalties: Patents are pending, but not issued, licensed, no royalties, no licensees., Research Funding; Bioline Rx: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding. Jaglowski:Juno: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; CRISPR: Consultancy; Novartis: Consultancy, Research Funding. Riedell:MorphoSys: Research Funding; Kite/Gilead: Research Funding, Speakers Bureau; Bayer: Consultancy, Speakers Bureau; Karyopharm Therapeutics: Consultancy; Celgene/Bristol-Myers Squibb: Consultancy, Research Funding; Novartis Pharmaceuticals: Consultancy, Honoraria, Research Funding. Perales:Celgene: Honoraria; MolMed: Membership on an entity's Board of Directors or advisory committees; Omeros: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bellicum: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Honoraria, Research Funding; Miltenyi Biotec: Research Funding; Merck: Consultancy, Honoraria; Nektar Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Cidara Therapeutics: Other; Servier: Membership on an entity's Board of Directors or advisory committees, Other; Medigene: Membership on an entity's Board of Directors or advisory committees, Other; NexImmune: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte Corporation: Honoraria, Research Funding. Kim:Gilead Sciences: Current equity holder in publicly-traded company; Kite, a Gilead Company: Current Employment. Kawashima:Kite, a Gilead Company: Current Employment; Gilead Sciences: Other: stock or other ownership . Yang:Kite, a Gilead Company: Current Employment. Rossi:Kite, a Gilead Company: Current Employment; Gilead Sciences: Current equity holder in publicly-traded company. Goyal:Kite, a Gilead Company: Current Employment. Neelapu:Legend Biotech: Other; Allogene Therapeutics: Other: personal fees, Research Funding; Cell Medica/Kuur: Other: personal fees; Incyte: Other: personal fees; Unum Therapeutics: Other, Research Funding; Adicet Bio: Other; Novartis: Other: personal fees; Bristol-Myers Squibb: Other: personal fees, Research Funding; Merck: Other: personal fees, Research Funding; Kite, a Gilead Company: Other: personal fees, Research Funding; Precision Biosciences: Other: personal fees, Research Funding; Poseida: Research Funding; Calibr: Other; Acerta: Research Funding; Takeda Pharmaceuticals: Patents & Royalties; Celgene: Other: personal fees, Research Funding; Pfizer: Other: personal fees; Karus Therapeutics: Research Funding; Cellectis: Research Funding; N/A: Other.
Author notes
Asterisk with author names denotes non-ASH members.