BACKGROUND
GVHD remains a frequent and serious complication of HSCT despite the decades-long use of standard immunosuppression, such as tacrolimus and methotrexate, as prophylaxis against GVHD. Preclinical models have shown that the timed infusion of donor-derived high-purity CD4+CD25+FOXP3+ regulatory T cells (Treg) preceding adoptive transfer of conventional T cells (Tcon) prevents GVHD and maintains anti-cancer immunity without the need of pharmacologic agents. Early clinical trials using purified Treg-engineered graft showed safety and feasibility, but more extensive clinical studies are needed to test scalability and efficacy. Orca-T is an industry-manufactured, precision-engineered CD34-selected, Treg-engineered graft made in a central GMP laboratory and distributed to multiple centers in the U.S. We present our early patient experience with Orca-T in an ongoing single center phase 2 trial and a multicenter Phase Ib trial and report a pre-planned evaluation of patients randomized to receive Orca-T plus single-agent GVHD prophylaxis (PPX) or Orca-T and no PPX.
METHODS
51 patients with high risk or active hematologic malignancies undergoing myeloablative conditioning were enrolled on two trials: a single-center Phase I/II (n=40, NCT01660607) and a multicenter Phase Ib (n=11; NCT04013685) study. Patients received CD34-selected cells infused with highly purified Treg (target dose: 3.0 x 106 cells/kg) followed 2 days later by the infusion of Tcon (3.0 x 106 cells/kg). Initial GMP manufacturing was demonstrated at Stanford (n=9 grafts; 2016 - 2019) and then transferred to Orca Bio in 2019 for scaled production.
We evaluated the 34 patients beyond dose escalation since July 2016 who received Orca-T grafts from either matched related (n=25) or unrelated (n=9) donors and single agent GVHD PPX with either tacrolimus (n=28) or sirolimus (n=6). For comparative analysis, we identified a contemporaneous SOC cohort at Stanford (n=138) with both matched related (n=79) and unrelated (n=59) transplant recipients who received unmanipulated PBSC products (median f/u 546 days) and methotrexate plus tacrolimus.
In April 2019, enrollment on a phase 2, stage 1 pre-planned subgroup of 24 patients were randomized to test whether all GVHD PPX could be removed: Orca-T plus either single-agent PPX (Arm 1, n=12) or no PPX (Arm2, n=12).
RESULTS
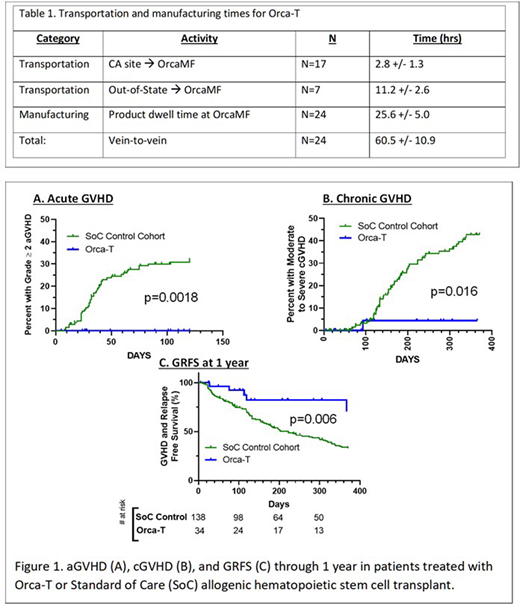
The Orca-T drug products were manufactured reliably with high Treg purity (93.8% +/- 3.1%) and a dose of 2.6 +/- 0.4 x 106 per kg (equivalent between arms and trials). Central lab turn-around times were 25.3 +/- 3.0 hours and all vein-to-vein times were less than 72 hrs (Table 1). For trial participants, there were no manufacturing failures, engraftment failures or treatment related mortality. Orca-T patients vs. SOC showed earlier neutrophil (median of 11 days vs. 14 days, p<0.0001 by Mann-Whitney U) and platelet engraftment (11 vs 17 days, p<0.0001) and a shorter hospital stay (15 vs 19 days, p=0.0002).
Patients across 4 clinical sites received Orca-T plus single agent GVHD PPX (n=34; median f/u 261 days) had an acute GVHD grade 2-4 incidence of 0% as compared to 33% in the SoC cohort (n=138, p=0.0018 by Log-rank Mantel-Cox test, Fig. 1). The rate of moderate to severe chronic GVHD for Orca-T patients was 4% vs. 44% in the SoC cohort (p=0.016).
In a preliminary subset of evaluable Orca- T patients, GVHD & relapse free survival (GRFS) at one year was higher for Orca-T patients vs SoC (69% versus 33%, p=0.006) while relapse and overall survival did not appear to differ. We observed no differences in infectious complications.
In patients randomized to PPX vs. no PPX, the incidence of aGVHD grade 2-4 was 0% in Arm 1 and 58% in Arm 2 (p <0.0001, Log-rank Mantel-Cox test), with 17% of these events being Grade 3-4. All GVHD in Arm 2 responded to steroids with no GVHD-related deaths.
CONCLUSIONS
Manufacture of high precision Orca-T Treg-engineered donor products were successfully scaled in a central GMP with reliable distribution to centers. Patients who received Orca-T and single agent PPX had no grade 2 or greater acute GVHD acute and very little chronic GVHD when compared to SOC. Patients randomized to Orca-T and no PPX showed an increased incidence of acute GVHD vs. those with Orca-T and single agent PPX. Engineered Treg grafts show promise to improve GFRS and other transplant outcomes. Orca-T has been granted RMAT status by the FDA and evaluation continues in an ongoing multicenter clinical trial.
Meyer:Orca Bio: Research Funding. Moroz:Orca Bio: Research Funding. Miklos:Novartis: Consultancy, Other: Travel support, Research Funding; Pharmacyclics: Consultancy, Other: Travel support, Patents & Royalties, Research Funding; Janssen: Consultancy, Other: Travel support; Miltenyi Biotec: Research Funding; Kite-Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding; Adaptive Biotech: Consultancy, Other: Travel support, Research Funding; Juno-Celgene-Bristol-Myers Squibb: Consultancy, Other: Travel support, Research Funding; Allogene Therapeutics Inc.: Research Funding. Shiraz:Kite, a Gilead Company: Research Funding; ORCA BioSystems: Research Funding. Muffly:Servier: Research Funding; Adaptive: Research Funding; Amgen: Consultancy. Rezvani:Pharmacyclics: Research Funding. Shizuru:Jasper Therapeutics, Inc: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees. Fernhoff:Orca Bio: Current Employment, Current equity holder in private company. Putnam:Orca Bio: Current Employment, Current equity holder in private company. McClellan:Orca Bio: Current Employment, Current equity holder in private company. Shaw:Orca Bio: Consultancy. McGuirk:Kite Pharmaceuticals: Consultancy, Honoraria, Research Funding, Speakers Bureau; Fresenius Biotech: Research Funding; Bellicum Pharmaceutical: Research Funding; Gamida Cell: Research Funding; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Astellas: Research Funding; Allo Vir: Consultancy, Honoraria, Research Funding; Novartis: Research Funding; Pluristem Ltd: Research Funding. Abedi:BMS, Gilead Sciences: Research Funding; AbbVie, BMS, Gilead Sciences, Seattle Genetics, Takeda: Speakers Bureau. Negrin:Magenta Therapeutics: Consultancy, Current equity holder in publicly-traded company; KUUR Therapeutics: Consultancy; Biosource: Current equity holder in private company; UpToDate: Honoraria; Amgen: Consultancy; BioEclipse Therapeutics: Current equity holder in private company.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract