Abstract
Platelets have long been known to play important roles beyond hemostasis and thrombosis. Now recognized as a bona fide mediator of malignant disease, platelets influence various aspects of cancer progression, most notably tumor cell metastasis. Interestingly, platelets isolated from cancer patients often display distinct RNA and protein profiles, with no clear alterations in hemostatic activity. This phenotypically distinct population, termed tumor-educated platelets, now receive significant attention for their potential use as a readily available liquid biopsy for early cancer detection. Although the mechanisms underpinning platelet education are still being defined, direct uptake and storage of tumor-derived factors, signal-dependent changes in platelet RNA processing, and differential platelet production by tumor-educated megakaryocytes are the most prominent scenarios. This article aims to cover the various modalities of platelet education by tumors, in addition to assessing their diagnostic potential.
Introduction
The historical interplay between platelets and cancer
Platelets are small (typically 1-3 μm in diameter) anucleate cells, derived from megakaryocytes (MKs) within the bone marrow and lung niches.1 During platelet production, MKs extend long cytoplasmic proplatelet extensions into blood vessel sinusoids, followed by a scission event that enables platelets to enter circulation. The majority of platelets remain quiescent during their 7- to 10-day lifespan. However, upon stimulation, platelets undergo drastic cytoskeleton-driven morphological changes, arrange homotypic aggregates via integrin coupling, and release a plethora of factors from specialized α-granules into their surrounding environment.2 These well-defined responses are essential to primary hemostasis, allowing platelets to adhere to damaged vasculature and act as “band-aids of the blood.” However, despite their clear importance to hemostasis, the inappropriate activation of platelets can promote various pathophysiologies, including thrombosis, inflammation, diabetes, and cancer.3-5
Cancer has long been known to influence platelets and hemostasis. In 1823, Jean-Baptiste Bouillaud first described cancer-associated blood clots,6 a finding that was followed by Trousseau’s seminal observations of spontaneous coagulation and increased risk of venous thromboembolism in cancer patients.7 Such findings led Trousseau to define migratory thrombophlebitis, commonly referred to as Trousseau syndrome, which he would later self-diagnose before succumbing to gastric cancer. Upon the subsequent discovery of platelets,8 clear correlations have been made between thrombocytosis (platelet counts, ≥450 × 109/L) and advanced malignant disease,9,10 indicating a supportive role for platelets in cancer progression. Similarly, thrombocytopenia (platelet counts, ≤150 × 109/L) induced by genetic or pharmacological murine models impairs tumor metastasis to the lung,11-14 supporting the involvement of platelets in metastatic disease. Over the last few decades, an extensive body of research has sought to characterize the mechanisms by which platelets promote various aspects of metastasis, including enhanced invasive capacity and intravasation, survival and transport within blood circulation, arrest at distant metastatic sites, and subsequent proliferation in competent organs.15-17 In addition to metastasis, platelets are thought to influence several other hallmarks of cancer, most notably evasion of the immune system, tumor-supportive inflammation, and neoangiogenesis.5,18,19 Such work exemplifies how cancer manipulates platelets to promote various aspects of disease progression.
An introduction to tumor-educated platelets
Tumor-educated cells modify their function in response to either local or systemic cues from the tumor parenchyma. This concept is commonly described with tumor-associated macrophages, which support angiogenesis, invasion, and metastatic spread through the release of various growth factors, cytokines, and proteases.20 Clear comparisons can be made between tumor-associated macrophages and platelets, which contain over 300 different cytokines within specialized α-granules, many of which are important regulators of wound repair following vascular injury, supporting growth, survival, neoangiogenesis, and remodeling of the extracellular matrix.21,22 These factors are typically released by activated platelets when preserving vascular integrity, but this process can also be hijacked by either tumor-derived platelet agonists or direct platelet-cancer cell interactions. Although we appreciate that tumor cell–induced platelet activation is an important mediator of cancer metastasis,15 many of the mechanisms adopted by tumor cells to activate platelets are also used in other (patho)physiological conditions such as hemostasis, thrombosis, sepsis, or aging.23-25 Clear distinctions can, however, be made between the RNA profiles of circulating platelets isolated from cancer patients, and that of healthy subjects or patients with other inflammatory conditions.26 Unlike the comparatively small proportion of platelets that locally interact with either circulating tumor cells (CTCs) or the tumor itself, this distinct population of circulating, inactivated platelets provide a readily available liquid biopsy as a novel cancer diagnostic and may themselves adopt novel biological functions.
In this article, we define tumor-educated platelets (TEPs) as functional cells in blood circulation with a distinct tumor-driven phenotype. Although the majority of studies investigating TEPs focus on altered RNA content, modifications to the platelet proteome will also be discussed. We also outline the mechanisms driving platelet education, which include (1) direct transfer and storage of tumor-associated factors, (2) tumor-induced alterations in platelet RNA processing, and (3) abnormal platelet production by MKs.
Mechanisms of platelet education
Uptake of protein
After red blood cells, platelets are the most abundant cells within blood circulation, possessing comprehensive endocytic machinery for the uptake and storage of various proteins.27,28 These features allow platelets to act as “sentinels of circulation,” which sample the systemic environment and retain factors originating from either malignant or nontransformed cells that collectively make up the tumor microenvironment.
Platelets can sequester plasma proteins that play important roles in tumor growth and neovascularization, including vascular endothelial growth factor (VEGF), basic fibroblast growth factor, and platelet-derived growth factor (PDGF).29 Colorectal cancer patients display increased platelet levels of VEGF and PDGF,30 which are likely endocytosed directly from plasma.31 Although little is known about the mechanisms underpinning protein uptake and storage into platelets, studies by Banerjee et al show that platelets use much of the basic internal machinery required for receptor-mediated endocytosis and endosomal trafficking.32,33 Although their experiments predominantly focused on fibrinogen, the mechanisms regulating endocytosis of other tumor-associated proteins and metabolites remain understudied.
Whether altered protein uptake by TEPs influences cancer progression is an important area of ongoing research. Kuznetsov et al demonstrated that proinflammatory and angiogenic cytokines secreted by aggressively growing “instigating” breast tumors are absorbed by platelets, which are in turn recruited to otherwise indolent “responding” tumors at distant anatomic sites to aid neovascularization.27 Such work shows that TEPs relay communications between distant tumor niches to orchestrate the eruption of indolent tumors into overt disease. TEPs can also mediate cross talk between tumors and the bone marrow niche to increase bone formation and turnover,34 a phenomenon associated with establishing a premetastatic milieu. Kerr et al demonstrated that platelets absorb several tumor-secreted proteins that encourage bone turnover, including transforming growth factor β-1 and matrix metalloproteinase-1, which are likely stored and released into the bone microenvironment via platelet α-granules.34 Importantly, Kerr et al confirmed that increased transforming growth factor β-1 and matrix metalloproteinase-1 in platelets originated from engrafted human tumor cells, as opposed to either de novo protein synthesis by MKs or the secretions of tumor-associated cells.34
RNA delivery
The presence of mutant or tissue-specific RNA transcripts within platelets demonstrates their ability to effectively traffic and store circulating tumor RNA. Prominent examples include EGFRvIII and PCA3 in platelets from patients with glioma or prostate cancer, respectively, as well as EML4-ALK rearrangements in patients with non–small cell lung carcinoma (NSCLC).35-37 Such markers are predominantly thought to enter platelets through fusion with tumor microvesicles or exosomes, although the presence of free RNAs in plasma implies that vesicle-independent mechanisms are also possible.35 How tumor microvesicles fuse and unload cargo into platelets has yet to be determined, as is the likelihood of these transcripts being translated into functional protein. Interestingly, mutant DNA variants of epidermal growth factor receptor associated with NSCLC, which can be detected in plasma, were not observed in the platelets of patients, despite the presence of EGFRvIII RNA transcripts.38 Such observations imply the selective delivery, packaging, and storage of RNA but not DNA within TEPs.
RNA processing
Despite being anucleate, platelets have long been known to undergo protein synthesis.39 Each platelet contains ∼2 fg of RNA, which is enriched further in younger reticulated cells before its decay or release during platelet activation.40,41 Advances in sequencing technologies have characterized a diverse range of RNA families within platelets, including unspliced pre-RNA, messenger RNA (mRNA), microRNA, ribosomal RNA, small nuclear RNA, transfer RNA, ribosomal RNA, long noncoding RNA (lncRNA), antisense RNA, and circular RNA, as well as a functional spliceosome for pre-RNA processing.40,42-45 Although changes in platelet RNA no doubt vary in part due to their MK precursors, thrombin-induced platelet activation and adherence to fibrinogen lead to splicing of pre-RNA into mature mRNA that is subsequently translated into functional proteins, including interleukin-1β, B-cell lymphoma-3, and tissue factor.42,46,47 Interestingly, bacteria and lipopolysaccharide induce RNA splicing of tissue factor transcripts in isolated platelets, likely explaining why mature tissue factor transcripts are found in the platelets of septic patients but not healthy subjects.48 In addition, fibrinogen binding to integrin αIIbβ3 on the platelet surface can induce de novo synthesis of P-selectin, an adhesion molecule thought to play an important role in tumor metastasis.49,50 These findings collectively demonstrate that RNA processing in response to platelet agonists from various (patho)physiologies can dynamically alter levels of mature RNA and protein within platelets.25,51,52
Calverley et al were the first to demonstrate differential RNA-splicing events in TEPs by isolating platelets from patients with metastatic lung cancer and subjecting their RNA to exon microarray analysis.53 In addition to finding 608 alternative splicing events in 424 genes, RNA expression was decreased in 197 of the 200 most prominently altered genes, which contrasts with a global increase in gene expression from platelets of septic patients.25 Similar observations were made by Best et al, who identified 1625 differentially spliced genes in platelets from NSCLC patients, with 927 genes displaying decreased splicing.26 Prominently spliced RNAs included CFL1, ACOT7, and ARPC1B, whereas splicing was significantly downregulated for DDX5, RPS5, and EEF1B2. Importantly, Best et al demonstrated that differential splicing was independent of age and various inflammatory conditions not associated with NSCLC.26 Although unconfirmed, abnormally spliced RNA in TEPs is likely a consequence of tumor-specific signals that lead to distinct pre-mRNA processing, although uptake or transfer of mature RNA from the surrounding environment is likely to contribute.35 The identity of such signals and how they mechanistically differ from those known to increase RNA splicing during platelet activation42,46,47 remain important unanswered questions. Whether spliced RNA in TEPs is translated into proteins that can influence platelet function also remains to be understood.
Although this article focuses on how solid tumors educate platelets, it is important to note that some hematological cancers can alter platelet RNA levels. Takagi et al demonstrated that plate-lets isolated from multiple myeloma patients have differential expression in ∼3000 genes.54 Unlike with NSCLC, expression was predominately upregulated (2200 genes) and platelets showed increased basal activation, implying that RNA modifications may reflect those seen with platelet activation by conventional agonists and not tumor-specific signals.42
In addition to differentially splicing mRNA, noncoding RNAs can also vary in TEPs. lncRNAs are a group of RNA molecules >200 nucleotides that do not encode protein, but instead regulate gene expression through epigenetic modifications, intracellular transport, RNA splicing, and transcriptional regulation.55 Paradoxically, the lncRNAs ZFAS1 and MAGI2-AS3 are downregulated in platelets isolated from patients with NSCLC, despite elevated ZFAS1 expression within lung tissue samples.38 Levels of small noncoding microRNAs involved in RNA silencing and posttranscriptional gene regulation are also altered in TEPs. Wang et al discovered that patients with nasopharyngeal carcinoma had selectively increased miR-34c-3p and miR-18a-5p in their platelets but not plasma, suggesting production within platelets or their MK precursors.56 Whether alterations in noncoding RNAs affect platelet gene expression remains an important question when determining molecular mechanisms driving the generation of TEPs (see Figure 1).
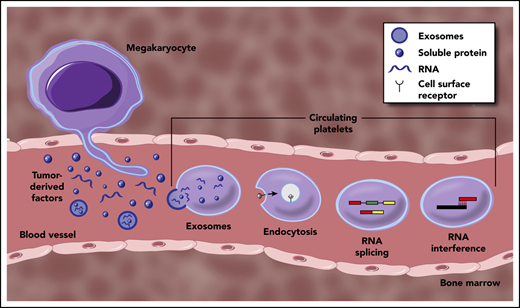
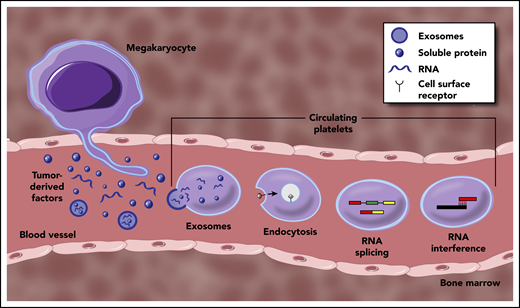
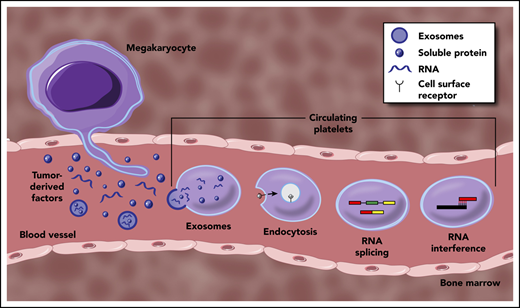
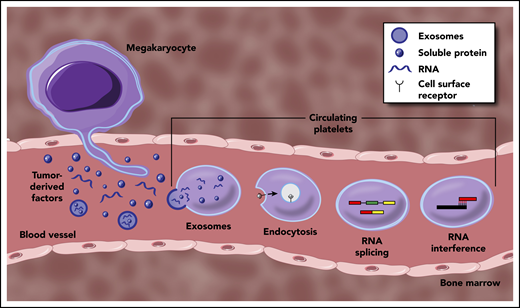
Mechanisms underpinning the generation of TEPs. Tumor-derived factors can educate mature platelets within blood circulation through several modalities. Direct mechanisms include the transfer of RNA or protein cargo via exosomes, or receptor-mediated endocytosis of soluble factors within circulation. Indirect education largely encompasses signal-dependent changes in RNA processing, most notably though alternative RNA splicing, but potentially through additional regulation of mature RNA transcripts. Tumor-derived factors may also alter the development and function of MKs within the bone marrow, resulting in a distinct platelet population that enters circulation.
Mechanisms underpinning the generation of TEPs. Tumor-derived factors can educate mature platelets within blood circulation through several modalities. Direct mechanisms include the transfer of RNA or protein cargo via exosomes, or receptor-mediated endocytosis of soluble factors within circulation. Indirect education largely encompasses signal-dependent changes in RNA processing, most notably though alternative RNA splicing, but potentially through additional regulation of mature RNA transcripts. Tumor-derived factors may also alter the development and function of MKs within the bone marrow, resulting in a distinct platelet population that enters circulation.
Tumor-educated MKs
Although circulating platelets acquire tumor-derived factors and undergo signal-dependent RNA modifications within the blood, alterations to platelet production by MKs within the bone marrow and lung niches are likely contributors to the distinct phenotype of TEPs. Cytokines increased in certain cancers such as granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, and interleukin-6 can increase megakaryopoiesis and platelet counts,57 often by upregulating thrombopoietin levels.58 Zaslavsky et al were the first to demonstrate that cancer not only affects the number of platelets produced but also platelet content due to MK alterations.59 In their study, bone marrow MKs displayed elevated expression of antiangiogenic thrombospondin-1, which was passed to their platelet progeny. They hypothesized that increased delivery of platelet thrombospondin-1 to the tumor site suppressed primary tumor growth, providing an example of negative feedback to the tumor microenvironment as a result of platelets produced by tumor-educated MKs.
During platelet production, MKs extend proplatelet protrusions into sinusoidal spaces, which separate into circulating platelets.60 Although our mechanistic understanding is limited, MKs selectively package RNA, proteins, and organelles into developing proplatelets,61 which can be altered by various pathophysiologies, including aging, sepsis, and systemic lupus erythematosus.23,52,61 For example, in age-associated inflammation, platelets from old mice have increased mitochondrial mass and number, resulting in a distinct metabolomic and respiratory profile.23 Similar experiments exploring differential organelle and RNA packaging by either bone marrow– or lung-resident MKs into platelets of cancer patients could provide insights into a novel mechanism of platelet education.
TEPs as a diagnostic tool
The current gold standard for cancer diagnosis still involves sampling suspect tissue via biopsy. This often-invasive approach provides limited spatial resolution to account for tumor heterogeneity and is usually conducted during advanced stages of disease, where treatment options become increasingly restricted.62 There is, therefore, great demand for a noninvasive biopsy for the early detection, profiling, and monitoring of various cancers. Such approaches have historically focused on specific tumor-derived proteins within blood circulation. However, more recent techniques include measuring circulating tumor-derived DNA or RNA, as well as the isolation and examination of CTCs or tumor extracellular vesicles.63 Although these developing approaches show promise, common limitations include the rarity and fragility of circulating tumor-derived DNA or RNA, and CTCs themselves, as well as complex and specialized methodology for their isolation.64 In contrast, platelets are one of the most abundant cells within circulation and have been routinely isolated for decades using simple, cost-effective procedures.65
Using platelets to support the diagnosis and monitoring of cancer is not a novel concept. Thrombocytosis and the platelet-to-lymphocyte ratio often correlate with increased incidence and poor survival outcomes.10,58,66,67 Similarly, mean platelet volume, a broad marker of platelet turnover, is also increased in early-stage but interestingly not late-stage lung cancer.68 However, despite their accessibility, such parameters are highly variable and can be influenced by other inflammation-associated diseases.69 Recent technological advances, however, enable one to look beyond basic platelet characterizations, such as number and size, to focus instead on protein or RNA as a diagnostic tool.
Platelet proteins in cancer diagnosis
The ability of platelets to sequester tumor-derived proteins within blood circulation has led to studies exploring whether changes in platelet protein content can be used as an early cancer diagnostic. Peterson et al were among the first to test this, demonstrating that VEGF, PDGF, and platelet factor 4 concentrations were higher in platelets but not the plasma of patients with colorectal cancer compared with controls.30 Similar approaches have also been explored in trials with lung cancer or head-of-pancreas cancer patients, where VEGF, PDGF, and platelet factor 4 concentrations were combined with platelet count and volume to provide a multiparameter platelet-based test for early-stage cancers.68 Broader proteomic approaches have also been adopted to quantify differential expression patterns between platelets isolated from patients with ovarian cancer compared with those with benign adnexal lesions, resulting in a biomarker panel that predicts early-stage disease with 88% sensitivity.70 Proteomic profiling of platelets can similarly identify patients with early-stage lung cancer or head-of-pancreas cancer.71 Interestingly, normalization of the platelet proteome following tumor resection highlights the potential of this approach not only as a diagnostic but also in monitoring therapy regimens and disease recurrence.71 However, further studies are required to establish whether such profiling is unique for cancer patients and not also observed in other inflammatory or age-associated diseases.
Platelet RNA leading the way
Platelet-derived RNA has recently been promoted as a putative diagnostic for various pathologies, including inflammation, sickle cell disease, acute myocardial infarction, and cancer.26,72-75 Calverley et al demonstrated the potential of platelet RNA as a cancer diagnostic tool for NSCLC in 2010.53 Since then, rapid advances in the sensitivity and depth of RNA sequencing have taken this concept to the forefront of translational medicine. Such progress is largely due to the work of Best et al, who developed a “pan-cancer” diagnostic called ThromboSeq, a sequencing platform that can identify spliced platelet RNA profiles from a single drop of blood.75 In brief, differentially spliced RNA profiles of 228 patients with various types of cancer (NSCLC, colorectal, glioblastoma, pancreatic, hepatobiliary, and breast) were compared with that of 55 healthy individuals, with a diagnostic accuracy of 96%. Location of the primary tumor was also determined with 71% accuracy, implying that differential alternative splicing by platelets depended on the type of cancer. In those studies, the educational programs in TEP RNA were principally influenced by tumor type as opposed to primary tumor progression or metastases. Although mutant RNA biomarkers produced by the primary tumor could not be detected in platelets, the global TEP mRNA profile was able to distinguish between patients with KRAS and EGFR mutant tumors, as well as patients with HER2-amplified or triple-negative breast cancer. Tumor-derived mutant V600E BRAF RNA is also undetectable in platelets isolated from melanoma patients,76 but EML4-ALK rearrangements can be detected in lung cancer patients with 80% accuracy.37 Such discrepancies warrant further investigation but imply that the diagnostic potential of TEPs largely lies in examining differential splice patterns of native RNA.
The initial work by Best et al in 2015 demonstrated the application of machine learning in TEP-based cancer diagnostics.75 Since then, increasingly robust analyses promote the accurate interpretation of complex transcriptomic data sets in TEPs.77 In a follow-up study, the ThromboSeq pipeline was adapted to include particle-swarm intelligence algorithms to generate biomarker selection panels that discriminate patients with early NSCLC from healthy individuals or, importantly, those with inflammatory conditions not associated with cancer.26 Similar machine-learning algorithms have detected TEPs in sarcoma patients with a diagnostic accuracy of 87%,78 and identified a 48-gene biomarker panel for NSCLC.79 Such findings illustrate how platelet RNA expression could be used to indicate various types and molecular subclasses of cancer to support routine clinical diagnosis and treatment regimens. We anticipate that publication of the ThromboSeq pipeline80 will allow further expansion and validation of machine learning–based RNA diagnostics from TEPs.
Emerging concepts
TEPs as a tool to predict treatment outcomes
In addition to providing an initial diagnostic, platelet RNA has the potential to coordinate and predict outcomes from therapeutic regimens. Park et al recently demonstrated that serial sampling of TEP RNA to detect EML4-ALK mutations can predict positive and adverse outcomes for ALK+ NSCLC patients taking alectinib or crizotinib.37 Serial monitoring of TEPs could also highlight novel acquired mutations during treatment that lead to chemoresistance. Although platelet levels of tumor-derived mutant RNA often lie beneath the detection limit of conventional reverse transcription polymerase chain reaction (PCR) and RNA-sequencing approaches,75,76 newer technologies such as droplet-digital PCR can analyze tumor RNAs within plasma with improved accuracy and sensitivity.81,82 Such advances are likely to improve the detection of mutant RNAs within TEPs.
Advances in RNA sequencing
The RNA studies cited in this article have largely been conducted using bulk analysis approaches, where platelets are indiscriminately isolated from whole blood before lysis and either sequencing or reverse transcription PCRs. Recent advances in single-cell RNA sequencing have already permitted a more detailed examination of MK heterogeneity.23,83 Although technical constraints including size and RNA quantity may limit current attempts to conduct single-cell RNA sequencing of platelets, such approaches would allow important questions to be addressed, including whether platelet education by tumor cells is homogenous.
Artificial education of platelets as a novel therapeutic strategy
Platelets typically support cancer metastasis. However, advances in biomedical engineering have enabled the ex vivo editing of platelets, which can be administered into circulation as a putative anticancer therapy. Antibodies targeting the immune checkpoint inhibitor programmed death ligand 1 (PDL1) have been expressed on platelets through either lentiviral-driven expression in cultured MKs or direct conjugation.84,85 Injection of these anti-PDL1–expressing platelets into mice reduced recurrent tumor growth and metastasis following surgical resection beyond that of unconjugated PDL1-targeting antibodies. In this study, efficacy was attributed to reduced anti-PDL1 clearance and the selective accumulation of platelets to sites of injury and inflammation, where tumor-induced platelet activation leads to the release of anti-PDL1–coated microparticles. Interestingly, administration of detergent-extracted “decoy” platelets into mice decreases metastatic tumor growth, possibly by disrupting conventional platelet–tumor cell interactions.86 Such studies demonstrate how artificially educated platelets can be used as a novel approach to treat cancer. Whether these preclinical findings can be translated into clinical observations remains to be seen.
Conclusions
Despite their anucleate nature and short lifespan, platelets are becoming ever more recognized for their capacity to be educated by various pathological stimuli. Importantly, the protein and RNA landscape of TEPs is dynamically transformed in patients with various types of cancer, even in the early stages of disease. Such a discrepancy highlights TEPs as a promising diagnostic, prognostic, and perhaps therapeutic tool in the search for novel approaches to confront malignancy.
Acknowledgment
This work was supported by the National Cancer Institute, National Institutes of Health (5R01CA200748-02).
Authorship
Contribution: H.G.R. and E.M.B. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elisabeth M. Battinelli, Brigham and Women’s Hospital, 4 Blackfan Circle, HIM 7th Floor, Boston, MA 02115; e-mail: embattinelli@bwh.harvard.edu.