Key Points
An abnormal venous calf muscle pump in the legs as measured by venous plethysmography is a risk factor for first VTE.
rCPF unilaterally or bilaterally is an independent predictor of all-cause mortality.
Abstract
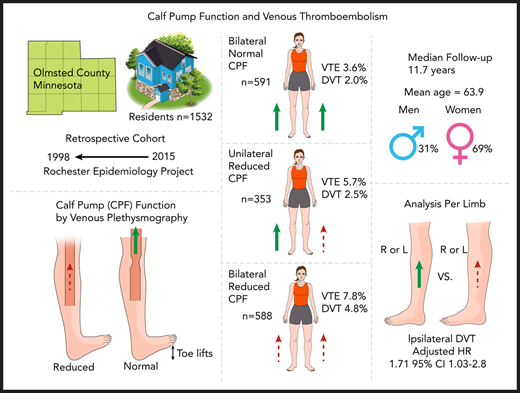
The calf muscle pump is a major determinate of venous return in the legs but has not been studied as a risk factor for venous thromboembolism (VTE). A population-based cohort study of Olmsted County, Minnesota residents was performed using calf pump function (CPF) measurements from venous plethysmography studies from 1998 to 2015. Patients with a history of VTE were excluded. Nursing validated VTE outcomes from the Rochester Epidemiology Project were identified after the index study date, and patients with reduced CPF (rCPF) were compared with patients with normal CPF. A total of 1532 patients with recorded CPF (28% air and 72% strain gauge plethysmography) were included; 591 (38.5%) had normal CPF, 353 (23.0%) had unilateral rCPF, and 588 (38.3%) had bilateral rCPF. Any VTE occurred in 87 patients (5.7%) after a median follow-up of 11.7 years (range, 0-22.0 years). Comparing patients with bilateral reduced to bilateral normal CPF, the unadjusted hazard ratio (HR) for incident VTE was 2.0 (95% confidence interval [CI], 1.2-3.4) and after adjusting for age, BMI, and Charlson Comorbidity Index, the HR was 1.68 (95% CI, 0.98-2.89). The adjusted HR for ipsilateral deep vein thrombosis was evaluated in 3064 legs comparing legs with reduced to normal CPF and was 1.71 (95% CI, 1.03-2.84). Mortality was significantly higher in both the bilateral (P < .001) and unilateral (P < .001) rCPF groups compared with normal CPF. Our results demonstrate that CPF is a risk factor for VTE in an otherwise low-risk ambulatory population and might be a useful component in risk stratification models.
Introduction
Venous blood from the lower extremities returns to the right side of the heart by a venous pressure gradient that is facilitated by 1-way valves and the venous muscle pump, of which the calf muscle pump is a major contributor.1-3 The volume of blood ejected from each calf with calf muscle contraction, or calf pump function (CPF), can be reliably measured with a series of toe lifts performed in an upright setting with venous plethysmography. Reduced CPF (rCPF) has been associated with chronic venous insufficiency and poor wound healing4 and has recently been identified as an independent predictor of all-cause mortality.5
Immobility is associated with venous stasis and is a well-known risk factor for deep vein thrombosis (DVT) and pulmonary emboli (PE).6 The current understanding of stasis and immobility as it relates to the formation of DVT and PE remains crude and is largely based on clinical intuition and clinical circumstances rather than a quantitative and personalized assessment. rCPF indicates the inability to efficiently expel blood from the lower extremities leading to venous stasis, even in ambulatory patients. rCPF is a plausible risk factor for venous thromboembolism (VTE), but this association has not been studied previously.
Using venous plethysmography data from the Mayo Clinic Gonda Vascular Laboratory, we evaluated the risk of DVT and PE in Olmsted County residents with rCPF compared with normal CPF.
Methods
Population
Olmsted County, Minnesota residents with no prior history of VTE who had venous plethysmography7 (strain gauge or air plethysmography) performed in the Mayo Clinic Vascular Laboratory between 1998 and 2015 were included in this study. The date of performance of venous plethysmography was considered as the index date. If >1 study was performed on an individual, the initial assessment was used as the index date for analysis. Only patients with CPF assessment and plethysmographically normal outflow bilaterally (indicating no obstructive DVT) were included. The Rochester Epidemiology Project (REP)8-10 captures virtually the entire population of Olmsted County and contains their demographic information, medical diagnoses, hospital admissions, surgical procedures, vital status, and validated VTE events.11 Patients with a history of VTE that was diagnosed before the index date and residents who denied Minnesota research authorization were excluded. The study was approved by the Mayo Clinic and Olmsted County Institutional Review Boards and conducted in accordance with the Declaration of Helsinki.
Covariates
Age at index study, sex, and body mass index (BMI; recorded within 3 years of index study) were extracted from the REP database and/or the Mayo Clinic Vascular Laboratory database. Comorbidities were extracted using ICD-9 codes for parameters included in the Charlson Comorbidity Index (CCI)12 if recorded in the 3 years before the index date (atrial fibrillation/flutter, myocardial infarction [MI], congestive heart failure [CHF], peripheral arterial disease [PAD], cerebrovascular disease [CVD], dementia, chronic pulmonary disease, peptic ulcer disease, diabetes with or without organ damage, hemiplegia, renal disease, liver disease, metastatic solid tumor, acquired immunodeficiency syndrome, rheumatologic disease, and other cancers).
Outcomes
The primary outcome was a composite of any incident VTE, including proximal and distal DVT and PE, as recorded in the REP database through 2015. The process for verification of VTE events in the REP has previously been described.11,13-16 Incident DVT events occurring in Olmsted County, Minnesota residents were objectively diagnosed by compression venous duplex ultrasonography, impedance plethysmography, computed tomographic venography, magnetic resonance imaging, venography, or pathology examination of thrombus removed at surgery or autopsy and were confirmed by study-trained registered nurses. PE within a segmental or larger artery was objectively diagnosed by a ventilation/perfusion lung scan interpreted as high probability for PE, computed tomographic pulmonary angiography, magnetic resonance imaging, pulmonary angiography, or pathology examination of thrombus at surgery or autopsy.
Venous plethysmography
Venous hemodynamics was assessed by plethysmography, which measures changes in the volume of a leg using pneumatic sensing cuffs or strain gauge sensors placed on the lower extremities.7,17 The study is done in 3 parts as summarized below, with detailed protocols provided in the supplemental Methods (available at the Blood Web site). First, maximal venous filling is achieved in the lower extremities, and venous outflow is measured via passive draining with the legs elevated. Patients with unexpected outflow obstruction are immediately referred for confirmatory lower extremity duplex ultrasound done on-site at the same visit to exclude acute DVT. Second, the patient is returned to the upright seated position allowing passive refilling of the lower extremities (passive dependent refill [PDR] volume). Third, CPF is assessed with subjects in a seated position, and the calf muscle pump is activated by having the patient perform toe lifts, which expels blood from the legs (exercise venous plethysmography [EVP]). After completion, passive refilling of the calf in the seated position is allowed to return to maximum filling (EVP refill volume). For air plethysmography, calf ejection fraction is calculated by dividing the EVP refill volume by the PDR volume (EVP/PDR × 100%). rCPF was defined as an ejection fraction of <45%. For strain gauge plethysmography, rCPF was defined as postexercise refill volume of <1.0 mL/100 mL. Venous insufficiency was categorized into normal, mild, moderate, or severe based on passive drain refill volumes and venous filling rates as previously described.18-20 In 2006 the vascular laboratory began a transition from strain gauge to air plethysmography. Data collected during the initial 4 years (2006-2010) after transitioning to air plethysmography was discarded due to either missing data and/or data irregularities. The weight limit for the venous plethysmography hemodynamic chair study is 300 pounds.
Analysis
Patients were divided into 3 groups based on CPF (bilateral rCPF, unilateral rCPF, and normal CPF). The χ2 test was used to compare categorical variables among the groups. Continuous variables were compared using the Kruskal-Wallis test. The date of the initial venous plethysmography study was the index date, and all subsequent incident VTE events were identified in individuals with the available follow-up data in the REP. Residents leaving the area were censored at the last known follow-up. Unadjusted hazard ratios (HRs) and Kaplan-Meier curves were created for the primary outcome of VTE and all-cause mortality for each group. Effect measure modification (interaction) was assessed by age (stratified at 65 years), BMI (stratified at 30), type of plethysmography (strain versus air), and sex (male versus female). To assess for possible dilution of the effect estimate for patients on anticoagulation or antiplatelet medication, a secondary analysis was performed calculating the unadjusted HR for VTE after excluding patients with baseline atrial fibrillation/flutter or CVD, PAD, MI, and CHF. A Cox proportional hazard model was then used to adjust for age, BMI (kg/m2), and the calculated CCI. A sensitivity analysis for this model was performed adding the degree of overall venous insufficiency (normal, mild, moderate, or severe) as classified by the worst extremity as well as the presence or absence of any degree of venous insufficiency (yes/no).
A secondary analysis based on each leg evaluated was also performed. Each leg was categorized as rCPF or normal CPF. Ipsilateral DVT outcomes were then evaluated per leg using unadjusted HRs to compare the groups. A Cox proportional hazard model was then used to adjust for age, sex, and BMI. These models were fit using sandwich estimators of the standard errors to account for multiple legs included for most of the patients.
Results
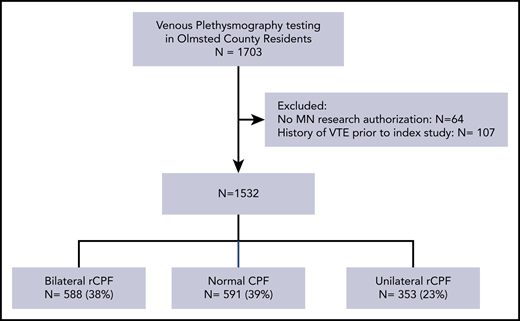
A total of 1703 Olmsted County residents had venous plethysmography studies performed in the study timeframe. Minnesota research authorization was either unavailable or declined by 64 patients, and 107 were excluded for a history of VTE preceding index study (Figure 1). A total of 1532 patients with recorded CPF (28% air and 72% strain gauge plethysmography) were included; 591 (38.5%) had normal CPF, 353 (23.0%) had unilateral rCPF, and 588 (38.3%) had bilateral rCPF. The median age was 63.9 years (standard deviation [SD], 18.4 years); 68.9% were female, and the median BMI was 28.8 kg/m2 (SD, 6.4 kg/m2). Patients with rCPF function were older on average than those with normal CPF (bilateral rCPF, 68.3 years; unilateral rCPF, 64.3 years; normal, 60.3 years; P < .001). Some medical comorbidities were more frequently observed in patients with unilateral and bilateral rCPF (Table 1). Patients with diabetes (without organ damage), renal disease, and metastatic solid tumors were more likely to have bilateral rCPF. A lower frequency of liver disease was observed in the bilateral rCPF group compared with normal CPF (2.0% vs 0.2%; P = .006). A trend toward a possible higher prevalence of CHF (P = .07) and PAD (P = .09) was seen in the bilateral rCPF group. Notably, we did not observe a difference in the median BMI across the groups (P = .66). The prevalence of patients with MI, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatologic disease, or other cancers was similar across the groups. The mean CCI was higher in the bilateral rCPF (2.4) and unilateral rCPF (1.8) groups compared with the normal CPF (1.5) group (P < .001).
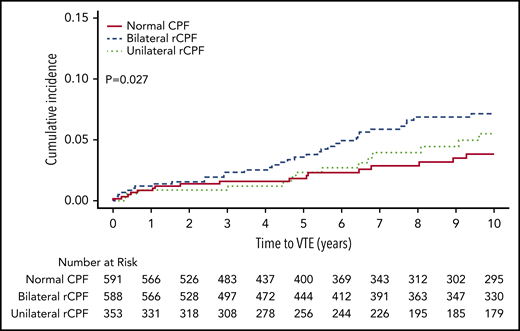
Any VTE occurred in 87 patients (5.7%) after a median follow-up of 11.7 years (range, 0-22.0 years; Table 2). The incidence rate (per 100 000 years) of VTE was 274 (95% confidence interval [CI], 157-445) in patients with bilateral normal CPF, 380 (95% CI, 208-580) in patients with unilateral rCPF, and 556 (95% CI, 391-776) in patients with bilateral rCPF. Isolated lower extremity DVT (excluding concurrent PE) occurred in 49 patients and PE ± DVT occurred in 38 patients. Bilateral rCPF compared with bilateral normal CPF was associated with an increased incidence of VTE (P = .008), DVT only (P = .02), proximal DVTs (P = .01), and higher mortality (P < .001), but not PE ± DVT (P = .13). Unilateral rCPF compared with bilateral normal CPF was not associated with an increased risk of VTE. Kaplan-Meier curves for VTE by CPF group are shown in Figure 2. The unadjusted HR for subsequent VTE was 2.0 (95% CI 1.2-3.4) and for DVT only was 2.2 (95% CI, 1.1-4.2) in individuals with bilateral rCPF compared with bilateral normal CPF. No significant interaction for the VTE outcome was identified based on the type of plethysmography study, age, sex, or BMI (P > .1 for each comparison). In a secondary analysis excluding patients with baseline atrial fibrillation/flutter (n = 91) due to possible anticoagulant use during the study period, the results for VTE remained unchanged (HR 2.0, 95% CI, 1.2-4.0). When patients with a possible indication for antiplatelet medications were excluded (n = 96; baseline history of MI, CHF, PAD, or CVD), the HR increased slightly to 2.4 (95% CI, 1.4-4.2). In a multivariable regression Cox proportional hazard model in individuals with bilateral rCPF compared with bilateral normal CPF, after adjusting for age, sex, BMI, and CCI, the findings were no longer statistically significant. The HR was 1.68 for subsequent VTE (95% CI, 0.98-2.89) and 1.60 for DVT only (95% CI, 0.8-3.2). A sensitivity analysis was performed including venous insufficiency as an additional variable in the model, which did not significantly change the results for the outcome of VTE when including graded venous insufficiency as classified by the worst extremity (HR, 1.62; 95% CI, 0.94-2.79) or presence or absence of any degree of insufficiency (HR, 1.63; 95% CI, 0.99-1.06).
For the per-leg analysis, 3064 legs (1532 patients each with 2 legs) were evaluated (Table 3). On the right, 42.2% (n = 646) had rCPF, and on the left, 45.0% had rCPF (P = .11). The pattern of the severity of venous insufficiency was similar in each leg, with 47.4% of right legs and 44.5% of left legs classified without appreciable venous insufficiency (P = .31). In total, there were 27 DVTs in the right leg (1.8% of right legs) and 43 DVTs in the left leg (2.8% of left legs; P = .053). The cumulative incidence of ipsilateral DVT was higher in the legs with rCPF (Figure 3), and the unadjusted HR was 2.0 (95% CI, 1.20-3.30). After adjustment, the HR for ipsilateral DVT was 1.71 (95% CI, 1.03-2.84).
Cumulative incidence of ipsilateral DVT per leg (right and left) by CPF.
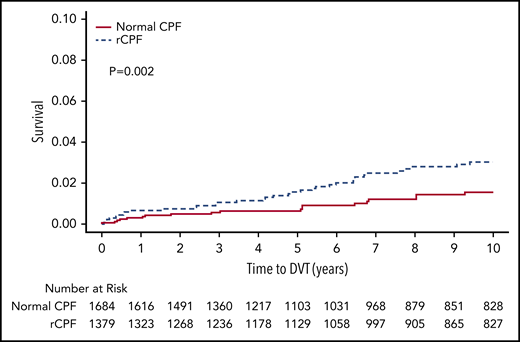
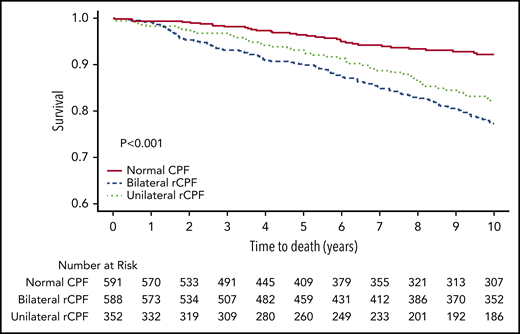
In the study time frame, 352 patients (23%) died, with a cumulative incidence of 11.0% in the normal CPF group, 22.4% in the unilateral rCPF group, and 35.4% in the bilateral rCPF group. The Kaplan-Meier survival curve is shown in Figure 4. Mortality was significantly higher in both the bilateral (P < .001) and unilateral (P < .001) rCPF groups compared with normal CPF (Table 2). The unadjusted HR for death was 2.6 (95% CI, 1.95-3.41) and after adjustment was 1.34 (95% CI, 1.00-1.79) in individuals with bilateral rCPF compared with bilateral normal CPF.
Discussion
In this population-based study of Olmsted County residents, rCPF, as measured by venous plethysmography, was associated with increased VTE, DVT specifically, and all-cause mortality. rCPF, either unilateral or bilateral, was observed in the majority of patients (61%) studied within this cohort, demonstrating the high prevalence of the condition in this predominately female population of referred patients. After multivariable adjustment for age, sex, BMI, and CCI, bilateral rCPF was not found to be an independent risk factor for VTE or DVT alone. However, when ipsilateral DVT was the outcome while examining each leg individually, rCPF remained an independent risk factor after adjustment. Both unilateral and bilateral rCPF compared with normal CPF were an independent predictors of all-cause mortality.
Underlying specific reasons for rCPF in this study are unclear. Older individuals and those with higher CCI scores were more likely to have bilateral or unilateral rCPF, indicating this is likely an acquired abnormality associated possibly with age-related sarcopenia/frailty or as a result of medical comorbidities limiting functional status. Other possible etiologies for rCPF might include renal impairment or right heart failure leading to hypervolemia and venous hypertension. Although most of the patients in this study were female, rCPF was not observed more frequently compared with men. One possible explanation for the higher frequency of females in the study is that venous plethysmography is often performed in patients with varicose veins and lower extremity edema, known to occur more frequently in females.21 Elevated BMI was not associated with rCPF in this study, despite its association with elevated venous hypertension and worsening chronic insufficiency.22 It is important to remember that more extreme elevated body weights would have been excluded, since the study weight limit was 300 lbs. Patients with outflow obstruction were also excluded from this study due to the possibility this might indicate an acute or chronic DVT. However, venous outflow obstruction solely based on obesity has been observed, and these patients might be at higher risk for VTE on that basis. Overall, the consistent effect in VTE outcomes across age, sex, and BMI indicates the functional impairment in rCPF, regardless of the underlying etiology, results in similar venous stasis as it relates to the risk for VTE.
Current thrombophilia evaluation and prediction for VTE is largely based on uncommon or rare inherited or acquired conditions that do not explain the majority of events which are often multifactorial. rCPF is a commonly identified abnormality on venous physiology testing that is a significant determinate of venous flow in the lower extremity and is a quantifiable marker for venous stasis in the lower extremities. Furthermore, rCPF provides a potential underlying and unifying pathophysiologic explanation that spans across many disease states and clinical circumstances and might at least partially explain multiple known epidemiologic associations between advancing age, BMI, and comorbidities with VTE.13 The CCI has been associated with VTE in cancer patients23 and ambulatory patients undergoing total hip arthroplasty24 and knee arthroplasty.25 Notably, in this study, Cox proportional hazard models with CPF did not show that sex, BMI, and CCI were independent predictors of incident VTE. Unlike comorbidities and inherited or acquired hypercoagulable states, which are persistent risk factors, calf muscle pump function can be improved with specific exercise programs,26,27 which means that it is a potentially modifiable VTE risk factor.
All-cause mortality was significantly associated with rCPF in this study. This finding, in a subset of patients with no venous insufficiency and no venous obstruction has previously been reported.5 In patients with bilateral rCPF compared with normal CPF, it was demonstrated that rCPF was a significant and additive contributor to mortality in patients with CHF. Results from this study further demonstrate that rCPF is an independent predictor of all-cause mortality, regardless of any associated venous insufficiency. Furthermore, this study also demonstrates a graded effect with increasing mortality with the number of legs involved. This finding argues against “generalized frailty” as an uncontrolled confounding variable, as this would presumably only result in bilateral rCPF. The correlation between increased mortality and increased VTE with rCPF is not a surprising finding, and we think that CPF could be an important tool in risk stratification models for both mortality and VTE risk.
In addition to the large sample size and comprehensive long-term follow-up data available via the REP on the cohort studied, the strengths of this study include high-quality outcomes of VTE, measured hemodynamic venous insufficiency, and multivariable adjustment. Recognized limitations of the study include retrospective design, potential missing data, and loss to follow-up due to subjects moving out of Olmsted County, Minnesota. Furthermore, a single measurement of CPF was used for this analysis, which may worsen or improve at a subsequent measurement depending on clinical circumstances. The reported HRs for VTE were possibly biased toward the null hypothesis (HR of 1.0) by the use of anticoagulant and antiplatelet medications for non-VTE indications. This uncontrolled variable was assessed via secondary analyses performed by excluding patients with select diagnosis codes. When doing so, a higher HR for VTE was observed after excluding patients with diagnosis codes demonstrating an indication for antiplatelet medications. It is also unknown to what extent this cohort of patients reflects the population more broadly, as all were referred for testing. While this creates a more homogenous population for cross-comparison, the high prevalence of rCPF observed is unlikely to be present in the population more broadly. When taking into account the median age of our cohort, the incident rate of VTE we observed in patients with normal CPF is very similar to what has been previously reported in other epidemiology studies,13 indicating no significant confounding on the basis of the referral for testing.
Conclusion
In this population-based study of Olmsted County residents with no prior VTE, rCPF as measured by venous plethysmography was associated with increased risk for VTE, particularly lower extremity proximal DVT. CPF may be a useful measurement to incorporate into VTE risk stratification models.
Deidentified data will be made available by emailing the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
There is a Blood Commentary on this article in this issue.
Acknowledgments
This publication was made possible through the support of the Eduardo G. Mestre and Gillian M. Shepherd, Clinician Career Development Award Honoring John T. Shepherd and the Rochester Epidemiology Project, which is supported by the National Institutes of Health, National Institute on Aging under award R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This paper is not under consideration elsewhere, and none of the paper’s contents have been previously published.
Authorship
Contribution: All authors were involved in the conception and design or analysis and interpretation of the data, drafting of the manuscript or revising it critically, and reading and approving of the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Damon E. Houghton, Division of Vascular Medicine, Department of Cardiovascular Diseases and Division of Hematology/Oncology, Department of Internal Medicine, Mayo Clinic, 200 1st St, Rochester, MN 55905; e-mail: houghton.damon@mayo.edu.