Key Points
The PEA is a technically simple nonradioactive functional HIT assay.
In a prospective blinded study, the PEA was highly accurate for the diagnosis of HIT classified by predefined clinicopathologic criteria.
Abstract
Heparin-induced thrombocytopenia (HIT) is a life-threatening, prothrombotic, antibody-mediated disorder. To maximize the likelihood of recovery, early and accurate diagnosis is critical. Widely available HIT assays, such as the platelet factor 4 (PF4) heparin enzyme-linked immunosorbent assay (ELISA) lack specificity, and the gold-standard carbon 14–labeled serotonin release assay (SRA) is of limited value for early patient management because it is available only through reference laboratories. Recent studies have demonstrated that pathogenic HIT antibodies selectively activate PF4-treated platelets and that a technically simpler assay, the PF4-dependent P-selectin expression assay (PEA), may provide an option for rapid and conclusive results. Based upon predefined criteria that combined 4Ts scores and HIT ELISA results, 409 consecutive adults suspected of having HIT were classified as disease positive, negative, or indeterminate. Patients deemed HIT indeterminate were considered disease negative in the primary analysis and disease positive in a sensitivity analysis. The ability of PEA and SRA to identify patients judged to have HIT was compared using receiver operating characteristic curve statistics. Using these predefined criteria, the diagnostic accuracy of PEA was high (area under the curve [AUC], 0.94; 95% confidence interval [CI], 0.87-1.0) and similar to that of SRA (AUC, 0.91; 95% CI, 0.82-1.0). In sensitivity analysis, the AUCs of PEA and SRA were also similar at 0.88 (95% CI, 0.78-0.98) and 0.86 (95% CI, 0.77-0.96), respectively. The PEA, a technically simple nonradioactive assay that uses ∼20-fold fewer platelets compared with the SRA, had high accuracy for diagnosing HIT. Widespread use of the PEA may facilitate timely and more effective management of patients with suspected HIT.
Introduction
Heparin-induced thrombocytopenia (HIT) is a life-threatening complication of heparin therapy caused by antibodies that recognize the platelet-specific CXC chemokine, platelet factor 4 (PF4), when it binds to heparin or other negatively charged macromolecules.1-5 Despite improvements in the understanding of HIT pathogenesis since it was recognized as a clinical entity several decades ago, patients suspected of having this condition continue to experience significant morbidity, particularly thrombosis, bleeding, and amputation, and ∼10% of cases end fatally.6 Once HIT is suspected, immediate discontinuation of heparin and institution of an alternate anticoagulant are mandatory. A false-positive test for HIT or a delay in excluding HIT needlessly subjects a patient to the considerable risks associated with treatment with a heparin alternative. Conversely, a false-negative HIT test can lead to catastrophic thrombosis or death.
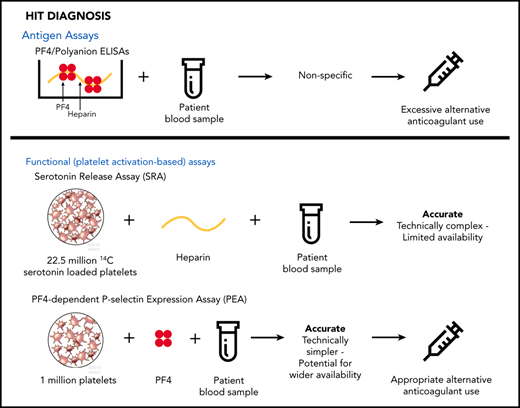
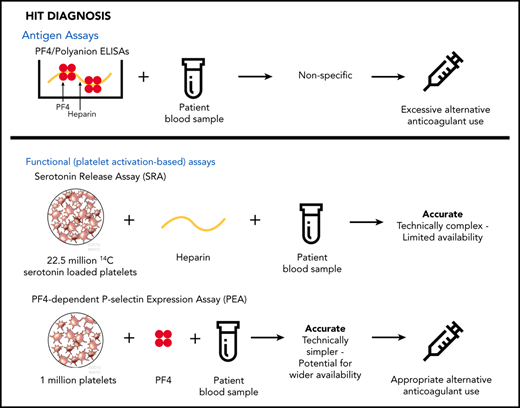
Pretest probability of HIT can be determined utilizing clinical scores such as the 4Ts score.7 Helpful information can also be gained by performing a relatively simple laboratory test, the PF4/polyanion enzyme-linked immunosorbent assay (ELISA), which detects antibodies found in virtually all patients with HIT.8 Because of its poor diagnostic specificity, however, the greatest value of the PF4 ELISA lies in the ability of a negative result to exclude the diagnosis. In contrast, many patients with a positive ELISA result do not, in fact, have HIT.
The serotonin release assay (SRA) detects pathogenic HIT antibodies preferentially and is widely considered to be the gold-standard laboratory test for HIT diagnosis.2 Thrombocytopenia and thrombosis in HIT result from the actions of a subset of heparin-induced pathogenic antibodies that activate platelets. Unfortunately, however, the SRA is technically demanding and is routinely available only through a few reference laboratories, precluding its impact on early decisions for patients with suspected HIT. An alternative platelet-activation assay, the PF4-dependent P-selectin expression assay (PEA), is based on the finding that incubation of platelets with PF4 primes them for recognition by pathogenic heparin-induced antibodies, which, upon binding to PF4-primed platelets, induce FcγRIIa (CD32)–dependent platelet activation and P-selectin (CD62p) surface expression.9 The PEA is technically simple, uses fewer platelets, and, unlike the SRA, requires no radioactive reagents, with the potential to make same-day results available to help with patient care decisions. Limited clinical studies have suggested that the accuracy of PF4-enhanced platelet activation assays for identification of patients who have HIT is comparable to or better than that of the SRA,10,11 whereas others have shown that use of PF4-treated platelets in functional platelet testing can detect pathogenic HIT antibodies earlier in the disease course.12,13 Here, we describe results of a multicenter, prospective, blinded study designed to compare the PEA and SRA in a large-scale clinical setting.
Methods
Population and 4Ts scoring
Consecutive adult (≥18 years of age) inpatients undergoing laboratory testing (ELISA) for suspected HIT between May 2016 and September 2017 were identified at the University of Washington (UW; Seattle, WA) and Mayo Clinic (Rochester, MN). The decision to perform HIT diagnostic testing was made by the primary clinical team; the involvement of a hematology specialist was not required for study inclusion. After exclusion of inadvertently collected samples, duplicate collections, or samples with inadequate volumes, 409 adult inpatients were included. Demographic information, type of heparin, and indication for use were recorded, and 4Ts clinical scores7 were calculated retrospectively by trained study personnel based upon the information available at the time that ELISA testing was ordered. Selective search functions and date limits were employed to avoid inadvertent viewing of laboratory results before calculation of the 4Ts scores. Information about outcomes was collected only after 4Ts scores had been determined and recorded. Follow-up data were recorded 30 days after HIT suspicion; the following information was abstracted: anticoagulant used for treatment of HIT or presumed HIT, hemorrhagic events (major or clinically relevant nonmajor bleeding), death, and cause of death. Major and clinically relevant nonmajor bleeds were defined as previously described.14 Coded samples with no associated clinical or laboratory data were sent to the central laboratory (Versiti, Milwaukee, WI) for PEA/SRA testing. The study was approved by the institutional review boards of UW, Mayo Clinic, and Medical College of Wisconsin.
Sample collection
Serum samples were collected for HIT ELISA testing as part of routine clinical care at the discretion of each patient’s primary provider and/or attending physician. Samples were processed according to institutional protocols, and unused material was shipped to the central laboratory for PEA/SRA testing. Both clinical centers coded samples before shipping but retained the link between code and patient so that test results could be correlated with clinical information.
HIT testing
Clinical sites performed immunoglobulin G (IgG)–specific HIT ELISAs (Zymutest HIA IgG; Hyphen Biomed [UW]; Lifecodes PF4 IgG; Immucor [Mayo Clinic]) and used manufacturer-recommended cutoffs for test positivity (optical density [OD] >0.3 [UW]; OD ≥0.4 [Mayo Clinic]). Samples were batch tested in the PEA and SRA by the central laboratory using the same target platelets in paired SRA-PEA runs so that patient samples were tested in both assays against the same donor platelets. The PEA and SRA were performed as detailed in the supplemental Materials (available on the Blood Web site). Just before commencement of testing, the laboratory of author A.P. observed that, when performed with lower concentrations of PF4, PEA may offer additional diagnostic utility.15 Therefore, 2 additional (lower) concentrations of PF4 were included in the study (10 [PEA10] and 3 μg/mL [PEA3]) as add-ons, and results for this testing are presented in the supplemental Materials.
Disease definition
To provide a basis against which to compare the test accuracy of the SRA and PEA, a clinicopathologic diagnostic scheme, involving a combination of 4Ts score (clinical) and ELISA OD (pathologic), was developed before study commencement8,12,16-18 to define whether HIT was very likely (positive), possible (indeterminate), or very unlikely (negative), as shown in Table 1. Given the desire for a stringent gold standard, indeterminate results were considered disease negative in the primary analysis. A sensitivity analysis was performed considering indeterminate results as disease positive, which is presented in the supplemental Materials.
Statistical analysis
Receiver operating characteristic (ROC) curves and corresponding confidence intervals [CIs] were calculated using the pROC function in the R statistical package,19 which uses trapezoids to calculate the area under the curve (AUC).20 CIs for the AUCs were calculated using Delong’s method.21 Positive thresholds for each assay were calculated by determining the point on the ROC curve that maximizes the sum of sensitivity and specificity when the minimum sensitivity was set at 85% in 1 analysis and minimum specificity was set at 95% in another. Concordance analysis was performed between the SRA and PEA, as well as between the activation assays and HIT ELISA.
Results
Characteristics of the patient population
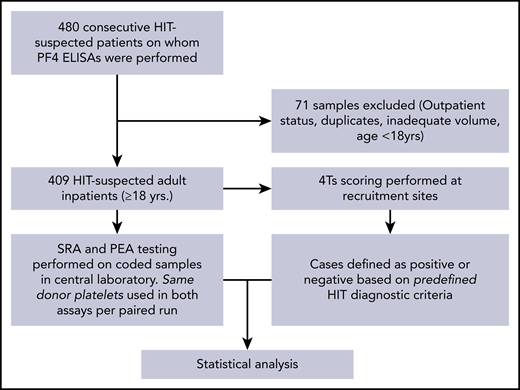
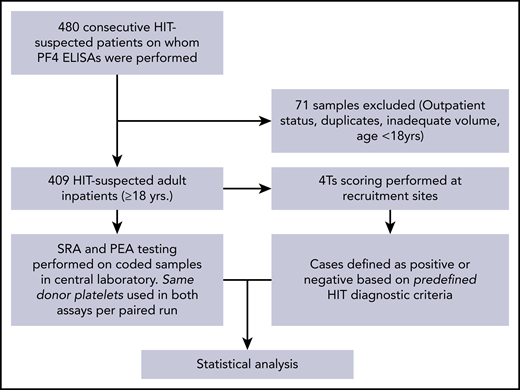
The study overview is provided in Figure 1. Remnant serum samples from consecutively suspected HIT patients from the 2 clinical sites were used in the study, with a total of 409 patients meeting study criteria. Patient characteristics are shown in Table 2. Patient ages ranged from 18 to 92 years (mean, 61 years), and 60% were male. Among patients receiving heparin prophylaxis, 85.3% received unfractionated heparin, 12.6% received low molecular weight heparin, and 1.7% received both unfractionated and low molecular weight heparin. Among patients who received heparin for venous thromboembolism treatment, 63.4% received unfractionated heparin, 31.7% received low molecular weight heparin, and 4.9% received both unfractionated and low molecular weight heparin. Major indications for heparin use were prophylaxis against venous thromboembolism (58.2%) and cardiac surgery (17.6%). Two hundred eighty-four patients (69.4%) had low 4Ts scores (1-3), whereas 98 (24%) and 27 (6.6%) had intermediate (4-5) or high (6-8) 4Ts scores, respectively. Forty-nine ELISA results were positive, and 360 were negative. Of the 409 patients, 17 were deemed to have HIT.
Study design. Consecutive samples suspected of HIT from two large tertiary care centers were evaluated in the PEA and SRA as shown. The central laboratory was blinded to clinical histories and HIT ELISA results. Disease state (HIT-positive) was pre-defined by clinico-pathologic diagnostic criteria.
Study design. Consecutive samples suspected of HIT from two large tertiary care centers were evaluated in the PEA and SRA as shown. The central laboratory was blinded to clinical histories and HIT ELISA results. Disease state (HIT-positive) was pre-defined by clinico-pathologic diagnostic criteria.
Test results and accuracy
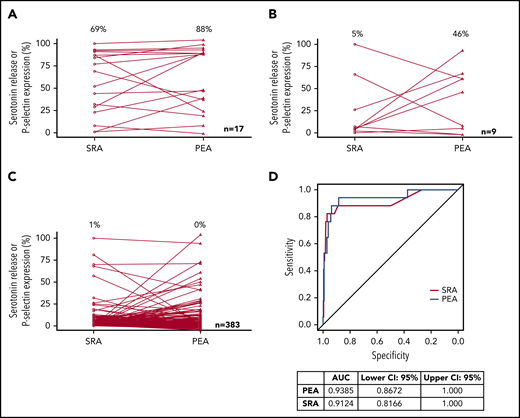
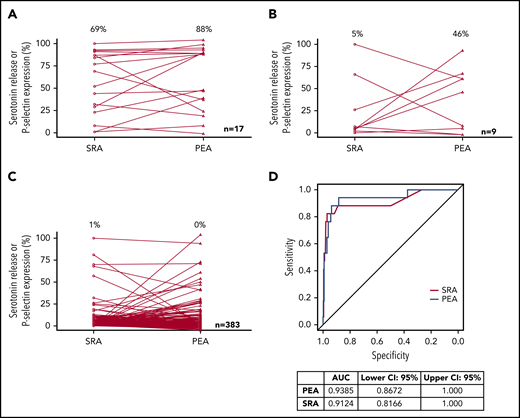
PEA and SRA results in samples that were deemed HIT+, HIT indeterminate, and HIT− are presented in Figure 2A-C, respectively. HIT patients classified as disease positive had median PEA and SRA results of 88% and 69%, respectively (Figure 2A). PEA and SRA results decreased significantly in HIT-indeterminate patients, with medians of 46% and 5%, respectively (Figure 2B). Figure 2C shows that a vast majority of samples from HIT-negative patients did not activate platelets in the PEA or the SRA. ROC curve analysis provides a means of estimating the extent to which a diagnostic assay correctly stratifies patients into disease-positive and -negative groups, and the area under an ROC curve (AUC) can be used to express the overall clinical accuracy of a test as a single number.22 Figure 2D shows the ROC analysis of the generated data. The diagnostic accuracy of the PEA was high (AUC, 0.94; 95% CI, 0.87-1.0), similar to that of the SRA (AUC, 0.91; 95% CI, 0.82-1.0; Figure 2D). Considering indeterminate patients as disease positive, 26 patients were deemed to have HIT, and the respective AUCs of the PEA and SRA were 0.88 (95% CI, 0.78-0.98) and 0.86 (95% CI, 0.77-0.96; supplemental Figure 1).
Test results and accuracy. PEA and SRA test results for HIT+ (A), HIT-indeterminate (B), and HIT− (C) samples are shown. Open circles and triangles refer to SRA and PEA test results, respectively. Median results are listed above each data set. (D) ROC testing for SRA and PEA and AUC estimates with CIs are presented.
Test results and accuracy. PEA and SRA test results for HIT+ (A), HIT-indeterminate (B), and HIT− (C) samples are shown. Open circles and triangles refer to SRA and PEA test results, respectively. Median results are listed above each data set. (D) ROC testing for SRA and PEA and AUC estimates with CIs are presented.
Sensitivity and specificity
Because of the high morbidity and mortality of this disease, high sensitivity is a key consideration in a frontline HIT diagnostic assay, a role the PEA could fulfill because of its technical simplicity. However, enhanced sensitivity at the cost of specificity would offer little advantage over currently used ELISAs. Therefore, positive test cutoffs were derived maximizing both sensitivity and specificity, with the minimum required sensitivity set at 85% (Table 3). Results show that, using a cutoff of 7.7%, PEA demonstrated high sensitivity (94%) and specificity (88%). An SRA cutoff of 7.5% was associated with sensitivity and specificity of 88% and 89%, respectively (Table 3). We then compared PEA performance with that of the SRA at or close to the conventionally used SRA+ cutoff. Twenty-one percent, close to the SRA cutoff of 20%, corresponded to a point on the SRA ROC curve that maximized the sum of sensitivity and specificity, with minimum required specificity set at 95% (Table 3). Cutoffs based on these criteria were derived for PEA, and sensitivity/specificity estimates were found to be similar to those of the SRA (Table 3).
Test concordance
Table 4 presents PEA-SRA concordance results using high specificity cut-offs (PEA 33.9%; SRA 21%) shown shown in Table 3. Negative concordance was high at 0.974, whereas positive concordance was 0.692 (Table 4). Clinical and HIT serologic profiles of patients with discordant results (ie, PEA+/SRA− and SRA+/PEA−) are shown in Table 5. Because the predefined HIT classification was based on 4Ts score at the time of HIT suspicion (and HIT ELISA obtained at that point) but lacked information on subsequent clinical course, detailed follow-up information was obtained on these discrepant cases that included platelet trending upon heparin cessation, as well as platelet response upon heparin reexposure (if reexposure occurred), to be able to confidently adjudicate whether the patient had HIT. Based on this evaluation, 5 of 10 PEA+/SRA− patients were deemed likely to have HIT (Table 5; supplemental Figure 2). Three of these patients were indeterminate, and 1 each was positive and negative by predefined criteria. In 1 patient, an SRA test obtained (for clinical purposes) a few days after the study sample was drawn seroconverted from negative to positive (patient 5; Table 5; supplemental Figure 2E), suggesting a false-negative test in the study sample. In addition, 2 of these 5 HIT+ patients were reexposed to heparin because of negative SRA results, and both experienced a decrease in platelet counts after exposure, confirming true HIT (patients 4 and 5; Table 3; supplemental Figure 2D-E). In the SRA+/PEA− patient group, 3 of 8 patients were deemed likely to have HIT (Table 5; supplemental Figure 3), 2 of whom were positive and 1 indeterminate based on predefined criteria. Positive and negative concordance of the SRA and PEA with the HIT ELISA, the frontline HIT assay in this study, were 0.449 and 0.989, and 0.490 and 0.989, respectively (supplemental Table 1).
Platelet-activating antibodies in HIT+ patients
In the 17 patients in the HIT+ group, the PEA and SRA were both negative in 2 patients (supplemental Table 2). Using follow-up clinical information, it was determined that only 1 of these 2 patients truly had HIT. The patient who did not have the disease underwent a second SRA (obtained for clinical purposes), which was also negative, and heparin was restarted with no adverse sequelae (supplemental Table 2).
PEA variants
The PEA was also performed with lower concentrations of PF4 (10 and 3 μg/mL), given recent suggestions of their potential utility in the diagnosis and follow-up of severely afflicted HIT patients.15 Supplemental Figure 4A-C shows that results of the PEA with smaller concentrations of PF4 were lower than those obtained with standard PEA. Similar to the PEA and SRA, ROC analysis showed high AUCs for both the PEA10 and PEA3 at 0.92 (95% CI, 0.84-1.0) and 0.95 (95% CI, 0.90-1.0), respectively (supplemental Figure 5).
Discussion
Early and accurate diagnosis is crucial for timely, appropriate management of HIT. In this blinded, prospective study, the technically simple PEA had high accuracy for the diagnosis of HIT. This was irrespective of the stringency of the HIT diagnostic classification used (ie, indeterminate results considered disease positive or negative), and these results suggest that the PEA could be used for rapid, accurate diagnosis.
HIT ELISAs are currently the first-line tests used to guide HIT management. The American Society of Hematology, as part of its Choosing Wisely stewardship campaign, has recognized the HIT ELISA as a test that should be questioned because of its low diagnostic specificity.23 A false-positive diagnosis of HIT can lead to substantial harm, because administering a nonheparin anticoagulant to a thrombocytopenic patient can increase the risk of bleeding.23,24 Our findings confirm a key flaw in the current diagnostic paradigm: 43 (88%) of 49 ELISA+ patients in our study were treated with nonheparin alternative anticoagulants, despite the fact that only 22 were ultimately confirmed to have HIT on the basis of the more specific SRA (supplemental Figure 6). Even if the 8 patients with a low 4Ts score were removed (who, according to expert recommendations, should never have undergone laboratory testing8 ), 37 (90%) of the remaining 41 patients were treated with alternative anticoagulation. Of these 37 patients, only 19 (51%) had serotonin release ≥20% in the SRA (data not shown).
The SRA is based on the premise that HIT antibodies bind to and activate heparin-treated platelets, whereas the PEA is fundamentally different in that HIT antibody-mediated activation of PF4-treated (not heparin-treated) platelets is assessed. In additional to being technically simple, a key advantage of the PEA is the requirement for a significantly smaller number of platelets per test (1 million vs ∼23 million for the SRA), such that a smaller volume of donor blood is required for performance of the assay. Consistent with prior studies, P-selectin expression was higher in the PEA relative to other versions of the assay using lower concentrations of PF4 that create fewer HIT antigen sites.15,25 Based on the results of this study, it may be concluded that the PEA variants performed with lower amounts of PF4 do not seem to provide additional diagnostic utility beyond that offered by the standard PEA. Our results also confirm the presence of the recently described entity of SRA− HIT,11,26,27 wherein samples from bona fide HIT patients are negative in the SRA but are positive in novel assays that use PF4-treated platelets, such as the PF4 SRA and the PEA. A likely explanation for this observation is that PF4-primed platelets have a lower threshold for activation and are able to detect HIT antibodies at the earliest signs of disease, when antibody titers may be lower,12,13 as highlighted by the patient presented in supplemental Figure 2E. In this study, we found the first few examples of SRA+/PEA− patients (Table 5), including 3 who were ultimately deemed to have HIT (supplemental Figure 3). We propose 2 possible explanations. First, the finding that PEA3 was positive at 12% and 37% in 2 of the 3 samples suggests the possibility of technical error in the PEA run; PEA results are typically much higher than those obtained in the PEA3 (supplemental Fig 4A-C). Alternatively, the discrepancy might be explained by differential activation thresholds for dense granule (serotonin) vs α granule (P-selectin) release with some but not other HIT antibodies.
Excessive laboratory testing for HIT is still a significant problem, as evidenced by the fact that a majority (69%) of patients in our study had low 4Ts scores. Only 2% of those with a low 4Ts score had platelet-activating antibodies in the SRA or PEA (supplemental Table 3), confirming the high negative predictive value of a low 4Ts score for HIT.28 For patients with an intermediate 4Ts score, the PEA+ and SRA+ rates were 12% and 10%, respectively. For those with a high 4Ts score, the positive rate was 41% for both assays (supplemental Table 3), consistent with previous estimates.28 Although significant educational interventions are needed to address the problem of excessive laboratory testing for HIT, 4Ts scores can be a challenge to calculate in medically complex patients, especially when transferred from other health care facilities.
Key strengths of this prospective study were that test platelets, known to vary in their reactivity to HIT antibodies,29 were from the same donors for paired PEA and SRA runs, thus allowing for a direct comparison of test results. In addition, the laboratory performing PEA and SRA testing was blinded to sample identity, which minimized confirmation bias. This contrasts with previous retrospective comparisons of PF4-enhanced HIT assays with the SRA that have used different platelet sources, thereby limiting the accuracy of comparisons made.10,27 Limitations include the lack of a true gold-standard assay to confirm or refute diagnosis. Although the investigators carefully designed a gold standard using clinical (4Ts score) and laboratory (HIT ELISA) criteria, some patients defined as HIT− or HIT indeterminate were found, based on clinical follow-up, to have HIT. However, the 3 strata defined for our analysis correlated with SRA results; SRA+ rates in HIT+, HIT-indeterminate, and HIT− groups were 82%, 33%, and 2%, respectively (supplemental Table 4). The PEA showed high accuracy relative to the SRA, irrespective of whether indeterminate results were considered disease negative (in the primary analysis) or positive (in the sensitivity analysis; Figure 2D; supplemental Figure 1). In this study, management was not directed by the results of these assays, and therefore, uncertainty exists about the ultimate impact of this novel assay on patient outcomes. Future studies utilizing the PEA in real time, either as a standalone assay or as part of a diagnostic algorithm in concert with the HIT ELISA, will be important to determine the ultimate role of this assay in the management of suspected HIT.
In summary, the PEA, a technically simple nonradioactive assay, demonstrated high diagnostic accuracy for the detection of pathogenic HIT antibodies. The PEA has the potential to facilitate rapid, accurate HIT diagnosis, minimize unnecessary use of nonheparin anticoagulant therapy, and improve outcomes among patients suspected of having HIT.
This work was presented as an oral abstract at the Annual Meeting of the American Society of Hematology, San Diego, CA, 1-4 December 2018.
For original data, please e-mail the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Allison Ujcich, Mia Sullivan, and Zaira Arteaga from the Platelet & Neutrophil Immunology Laboratory, Versiti Wisconsin, for logistic and technical assistance for SRA studies.
This work was supported, in part, by National Institutes of Health, National Heart Lung and Blood Institute grants HL133479 (A.P.), HL013629 (R.H.A.), and 2T32HL007093-41 (Hematology Training Grant; B.S.B.).
Authorship
Contribution: B.S.B., D.M.W., R.H.A., D.A.G., and A.P. were involved in study design, analysis, data interpretation, and manuscript writing; C.G.J. performed most PEA studies; S.M.P. performed PEA testing and coordinated sample management; B.R.C. oversaw SRA testing; B.S.B., D.M.W., P.R.K., and D.A.G. reviewed patient histories and performed clinical scoring; M.W.R. helped with disease classification; D.E.G. performed the statistical analyses; C.G.J., D.W.B., R.S., R.R.L., R.K.P., D.C., and D.E.S. provided helpful advice; and all authors critiqued and approved the final version of the manuscript.
Conflict-of-interest disclosure: C.G.J. reports issued patents on HIT diagnosis, with patents assigned to Versiti, Inc., and equity ownership and employment in Retham Technologies. B.R.C. reports personal fees from Ionis Pharmaceuticals and RallyBio, outside the submitted work, and an issued patent on HIT diagnosis, with the patent assigned to Versiti, Inc., outside the submitted work. D.W.B. reports issued patents on HIT diagnosis, with patents assigned to Versiti, Inc. R.K.P. reports honoraria for advisory board participation from CSL Behring, Genentech, Bayer Healthcare AG, HEMA Biologics, Instrumentation Laboratory, and Merck, outside the submitted work. R.H.A. reports issued patents on HIT diagnosis, with patents assigned to Versiti, Inc., and reports serving on the advisory board of Retham Technologies. D.A.G. reports grants from Incyte, personal fees and nonfinancial support from Janssen, personal fees from Seattle Genetics, and grants from Daiichi Sankyo, outside the submitted work. A.P. reports issued patents on HIT diagnosis, with patents assigned to Versiti, Inc., reports consulting fees and equity ownership in Retham Technologies, and reports serving on the advisory board of Veralox Therapeutics and as a consultant for Terumo BCT, outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: David A. Garcia, Department of Medicine, University of Washington, Seattle, WA 98109; e-mail: davidg99@u.washington.edu; or Anand Padmanabhan, Department of Laboratory Medicine & Pathology, Mayo Clinic, Rochester, MN 55905; e-mail: padmanabhan.anand@mayo.edu.
REFERENCES
Author notes
B.S.B. and D.M.W. contributed equally to this work.